Fractionation effect of iron isotope during magmatism and its indication of submarine basalt formation process
-
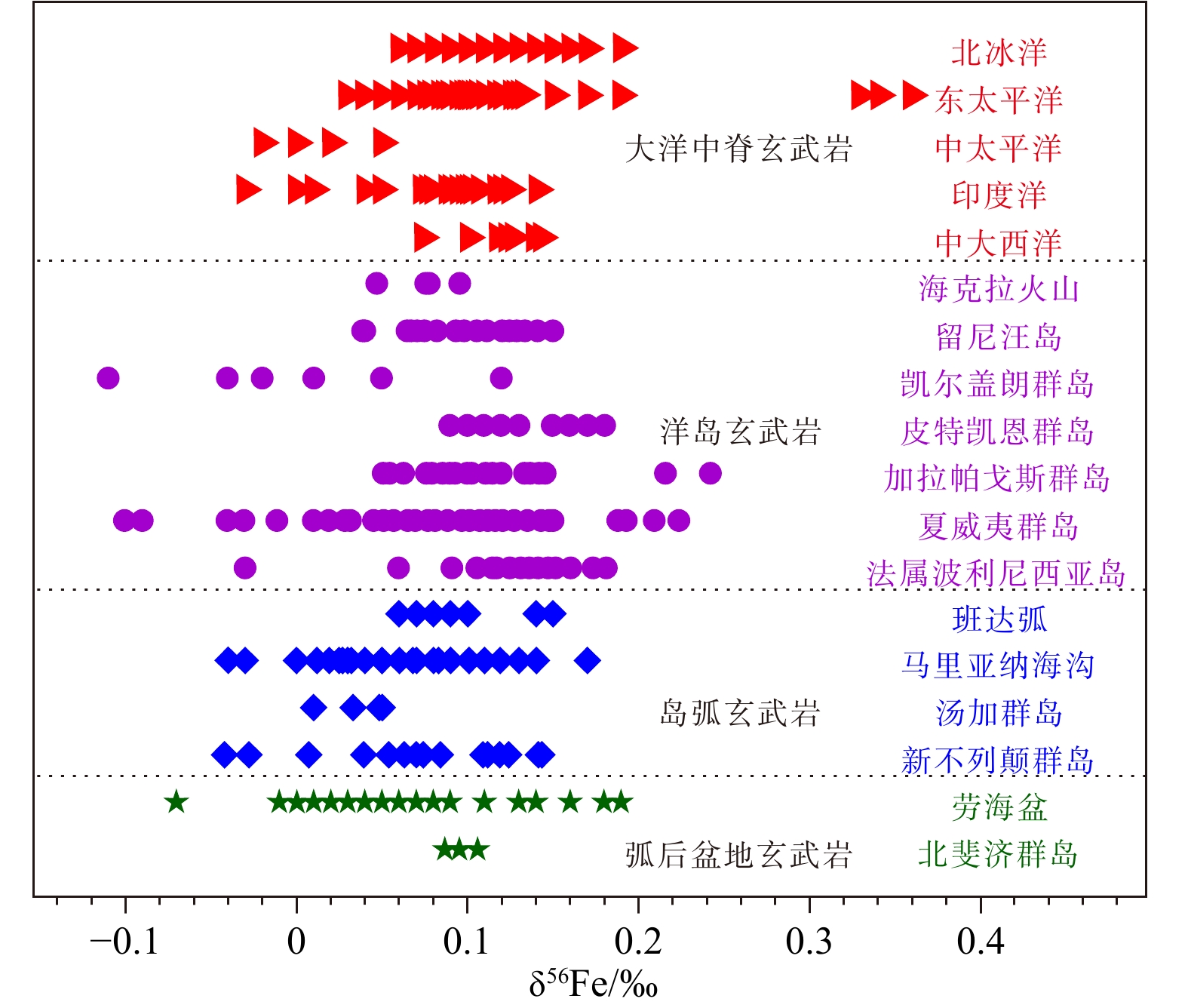
摘要: Fe是火成岩中丰度最高的变价元素,也是重要的成矿元素,主要以Fe2+或Fe3+价态赋存于固(矿物)、液(流体)相中,并全程参与岩浆作用过程和各种成矿作用。随着测试分析技术(如MC-ICPMS)的发展,Fe等非传统稳定同位素组成分析成为可能,并在最近十几年中被成功应用于岩浆物源追溯、结晶演化过程示踪和成矿作用分析等重要地质作用过程的研究。本文在分析了Fe同位素在岩浆作用过程中分馏效应的基础上,总结了Fe同位素组成在示踪海底玄武质岩浆(MORB、OIB、IAB和BABB等)作用过程研究的最新成果,并探讨了在应用Fe同位素组成示踪海底岩浆作用过程中所存在的主要问题。综合分析结果表明,火成岩中的Fe同位素分馏效应不仅受岩浆源物质部分熔融、岩浆扩散、流体出溶和结晶分异等作用过程的影响,而且还受到同化围岩物质、海底蚀变等作用的影响;由于Fe同位素分析技术(方法)至今仍待进一步完善,已有数据有限且需甄别去伪,因此在利用Fe同位素组成分析或恢复岩浆物源及作用过程时,仍需谨慎;于当前亟需建立完整可靠的Fe同位素示踪体系,这就需要在近期的工作中,尽可能多地选取代表不同构造环境和不同岩石类型的合适样品、获取(积累)更多原始(未经改造或蚀变)样品的精细分析数据,同时在利用Fe同位素示踪海底岩浆作用过程中还需注重多元数据的结合或相互佐证。Abstract: Fe is the most abundant variable-valence element in igneous rocks, and is also an important mineralizing element, mainly in the solid (mineral) and liquid (fluid) phases in Fe2+ or Fe3+ valence state, and participates in magmatic processes and various mineralization throughout. With the development of test analytical techniques (e.g. MC-ICPMS), the analysis of non-traditional stable isotope compositions such as Fe has become possible and has been successfully applied to the study of important geological processes such as magma source tracing, tracing of crystallization evolutionary processes and mineralization analysis in the last decade or so. Based on the analysis of the fractionation effect of Fe isotopes during magmatism, this paper summarized the latest results of Fe isotope composition studies in tracing the action of seafloor basaltic magmas (MORB, OIB, IAB and BABB, etc.) and discussed the main problems in the application of Fe isotope composition in tracing the action of seafloor magmas. The results of the comprehensive analysis show that the Fe isotope fractionation effect in igneous rocks is influenced not only by the processes of partial melting of magma source material, magma diffusion, fluid exsolution and crystallization differentiation, but also by the assimilation of surrounding rock material and seafloor alteration. Since Fe isotope analysis techniques (methods) have yet to be further refined, and the available data are limited and need to be screened for artifacts, caution is still needed when using Fe isotope compositions to analyze or recover magmatic sources and processes. It is urgent to establish a complete and reliable Fe isotope tracing system, which requires the recent work to select as many suitable samples as possible representing different tectonic environments and different rock types, to obtain (accumulate) more fine analytical data of original (unmodified or altered) samples, and to pay attention to the combination or mutual corroboration of multiple data in the process of using Fe isotope tracing for seafloor magmatism.
-
Key words:
- iron isotope /
- fractionation effect /
- isotopic tracing /
- submarine magmatism
-
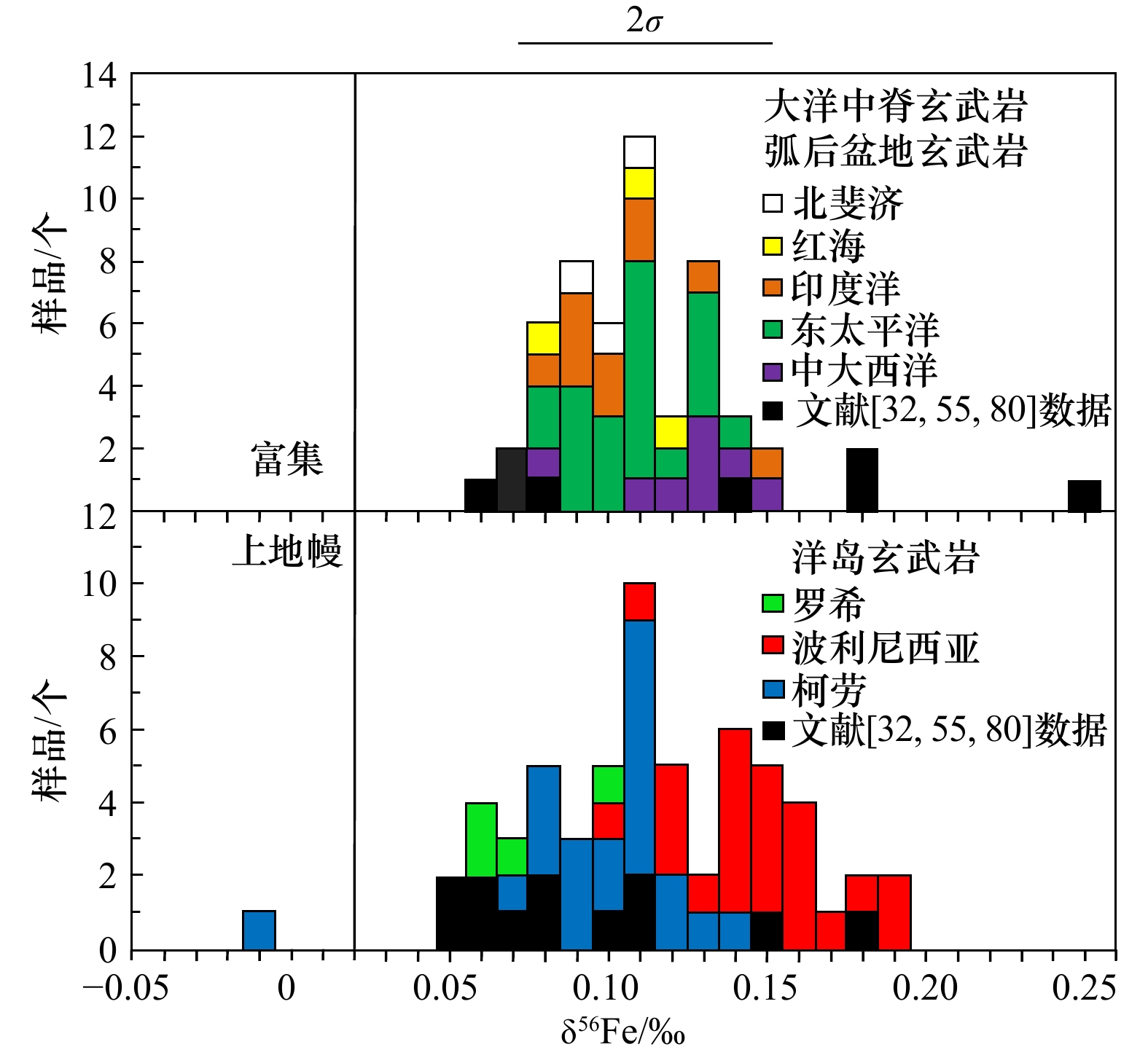
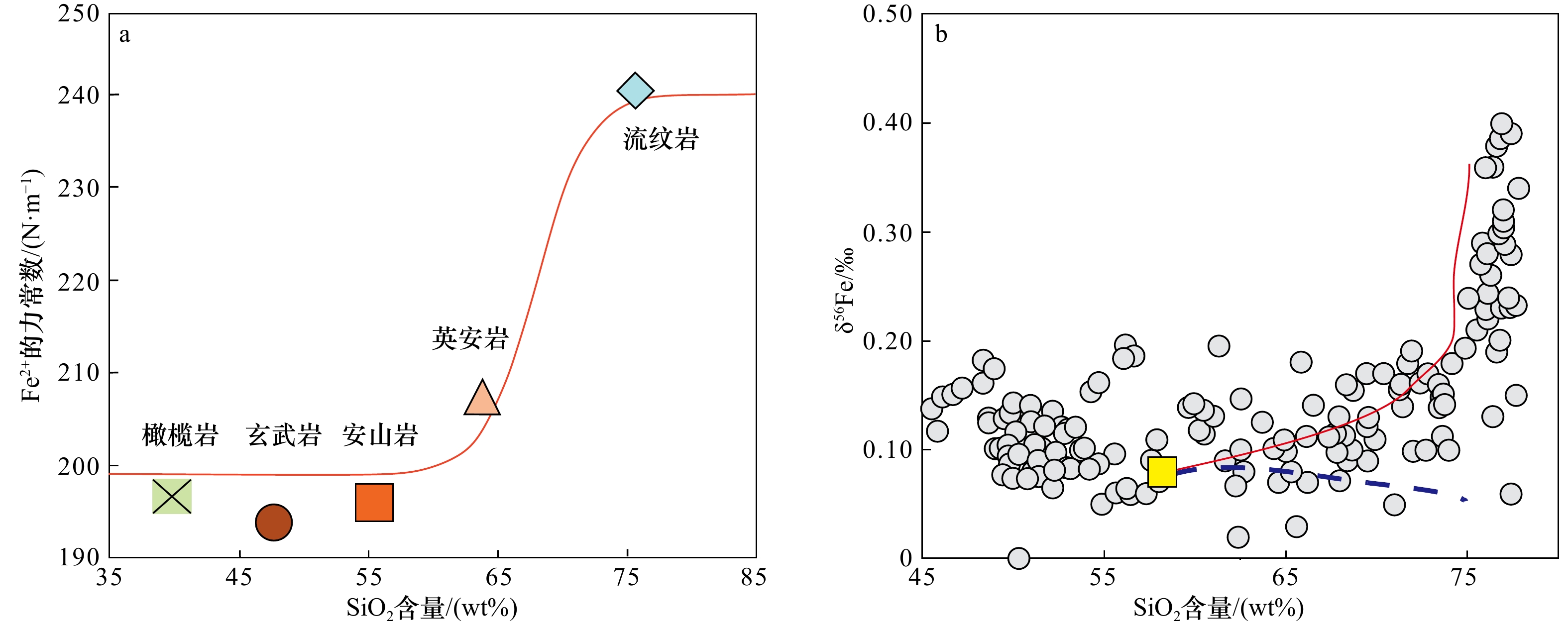
图 1 火山岩中Fe同位素分馏的控制(据文献[59])
Fe2+的力常数在SiO2含量为(65~75)wt%之间出现突变(图1a),这种变化可以部分解释硅酸岩的δ56Fe值在SiO2含量高于70 wt%时快速增加的原因(图1b);b中灰色圆圈是由文献数据绘制的点,红色曲线是使用rhyolite-MELTS软件模拟安山岩熔体分离结晶计算得出,蓝色虚线显示了残余熔体的同位素演化
Fig. 1 Control of iron isotope fractionation in siliceous rocks (from reference [59])
The force constant of Fe2+ shows an abrupt change between 65 wt% and 75 wt% of SiO2 content (Fig. 1a), which can partly explain the rapid increase of δ56Fe values of silicate rocks above 70 wt% SiO2 content (Fig. 1b); in b, gray circles are points drawn from literature data, red curves are calculated using rhyolite-MELTS simulation for the fractional crystallization of andesite melt, and blue dotted lines show the isotopic evolution of the residual melt
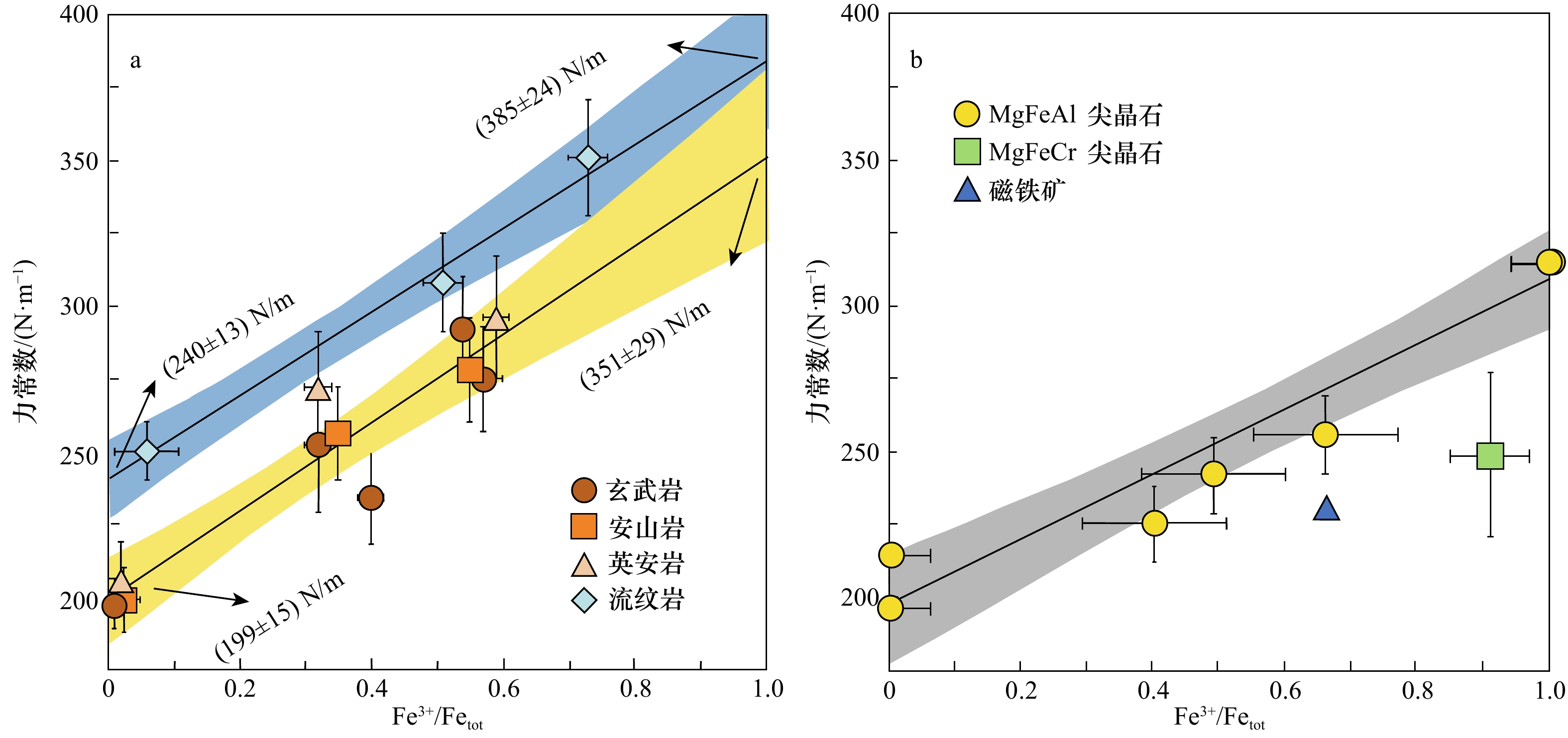
图 2 玄武岩、安山岩、英安岩、流纹岩玻璃(a)和尖晶石(b)的力常数测量值
该力常数测量值是铁氧化还原状态的函数(数据取自文献[59, 86]);在高温平衡时,Fe同位素分馏与力常数成正比;1 000 ln β=2 904<F>/T2
Fig. 2 Force constant measurements of basalt, andesite, dacite, rhyolite glasses (a) and spinels (b)
The force constant measurements are function of the redox state of iron (data from references [59, 86]); at high temperature equilibrium, iron isotopic fractionation is directly proportional to the force constant; 1 000 ln β=2 904<F>/T2
-
[1] Zhu X K, Guo Y, Williams R J P, et al. Mass fractionation processes of transition metal isotopes[J]. Earth and Planetary Science Letters, 2002, 200(1/2): 47−62. [2] 张宏福, 汤艳杰, 赵新苗, 等. 非传统同位素体系在地幔地球化学研究中的重要性及其前景[J]. 地学前缘, 2007, 14(2): 37−57. doi: 10.3321/j.issn:1005-2321.2007.02.004Zhang Hongfu, Tang Yanjie, Zhao Xinmiao, et al. Significance and prospective of non-traditional isotopic systems in mantle geochemistry[J]. Earth Science Frontiers, 2007, 14(2): 37−57. doi: 10.3321/j.issn:1005-2321.2007.02.004 [3] Richter F M, Dauphas N, Teng Fangzhen. Non-traditional fractionation of non-traditional isotopes: evaporation, chemical diffusion and Soret diffusion[J]. Chemical Geology, 2009, 258(1/2): 92−103. [4] 朱祥坤, 王跃, 闫斌, 等. 非传统稳定同位素地球化学的创建与发展[J]. 矿物岩石地球化学通报, 2013, 32(6): 651−688.Zhu Xiangkun, Wang Yue, Yan Bin, et al. Developments of non-traditional stable isotope geochemistry[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2013, 32(6): 651−688. [5] 刘耘. 非传统稳定同位素分馏理论及计算[J]. 地学前缘, 2015, 22(5): 1−28.Liu Yun. Theory and computational methods of non-traditional stable isotope fractionation[J]. Earth Science Frontiers, 2015, 22(5): 1−28. [6] Zhu Dan, Bao Huiming, Liu Yun. Non-traditional stable isotope behaviors in immiscible silica-melts in a mafic magma chamber[J]. Scientific Reports, 2015, 5(1): 17561. doi: 10.1038/srep17561 [7] Young E D, Manning C E, Schauble E A, et al. High-temperature equilibrium isotope fractionation of non-traditional stable isotopes: experiments, theory, and applications[J]. Chemical Geology, 2015, 395: 176−195. doi: 10.1016/j.chemgeo.2014.12.013 [8] Watkins J M, DePaolo D J, Watson E B. Kinetic fractionation of non-traditional stable isotopes by diffusion and crystal growth reactions[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 85−125. doi: 10.2138/rmg.2017.82.4 [9] Teng Fangzhen, Wang Shuijiong, Moynier F. Tracing the formation and differentiation of the Earth by non-traditional stable isotopes[J]. Science China Earth Sciences, 2019, 62(11): 1702−1715. doi: 10.1007/s11430-019-9520-6 [10] 韦刚健, 黄方, 马金龙, 等. 近十年我国非传统稳定同位素地球化学研究进展[J]. 矿物岩石地球化学通报, 2022, 41(1): 1−44.Wei Gangjian, Huang Fang, Ma Jinlong, et al. Progress of non-traditional stable isotope geochemistry of the past decade in China[J]. Bulletin of Mineralogy, Petrology and Geochemistry, 2022, 41(1): 1−44. [11] Schoenberg R, von Blanckenburg F. Modes of planetary-scale Fe isotope fractionation[J]. Earth and Planetary Science Letters, 2006, 252(3/4): 342−359. [12] Wang Kun, Moynier F, Dauphas N, et al. Iron isotope fractionation in planetary crusts[J]. Geochimica et Cosmochimica Acta, 2012, 89: 31−45. doi: 10.1016/j.gca.2012.04.050 [13] Sossi P A, Nebel O, Foden J. Iron isotope systematics in planetary reservoirs[J]. Earth and Planetary Science Letters, 2016, 452: 295−308. doi: 10.1016/j.jpgl.2016.07.032 [14] Elardo S M, Shahar A, Mock T D, et al. The effect of core composition on iron isotope fractionation between planetary cores and mantles[J]. Earth and Planetary Science Letters, 2019, 513: 124−134. doi: 10.1016/j.jpgl.2019.02.025 [15] 赵新苗, 朱祥坤, 张宏福, 等. Fe同位素在地幔地球化学研究中的应用及进展[J]. 岩石矿物学杂志, 2008, 27(5): 435−440. doi: 10.3969/j.issn.1000-6524.2008.05.008Zhao Xinmiao, Zhu Xiangkun, Zhang Hongfu, et al. Applications of Fe isotopes to tracing mantle processes[J]. Acta Petrologica et Mineralogica, 2008, 27(5): 435−440. doi: 10.3969/j.issn.1000-6524.2008.05.008 [16] Shahar A, Young E D, Manning C E. Equilibrium high-temperature Fe isotope fractionation between fayalite and magnetite: an experimental calibration[J]. Earth and Planetary Science Letters, 2008, 268(3/4): 330−338. [17] 朱祥坤, 孙剑, 王跃. 岩浆过程中铁同位素的地球化学行为[J]. 地球科学与环境学报, 2016, 38(1): 1−10. doi: 10.3969/j.issn.1672-6561.2016.01.001Zhu Xiangkun, Sun Jian, Wang Yue. Fe isotope geochemistry of magmatic system[J]. Journal of Earth Sciences and Environment, 2016, 38(1): 1−10. doi: 10.3969/j.issn.1672-6561.2016.01.001 [18] Johnson C, Beard B, Weyer S. High-temperature Fe isotope geochemistry[M]//Iron Geochemistry: An Isotopic Perspective. Cham: Springer, 2020: 85−147. [19] Johnson C M, Skulan J L, Beard B L, et al. Isotopic fractionation between Fe(III) and Fe(II) in aqueous solutions[J]. Earth and Planetary Science Letters, 2002, 195(1/2): 141−153. [20] Welch S A, Beard B L, Johnson C M, et al. Kinetic and equilibrium Fe isotope fractionation between aqueous Fe(II) and Fe(III)[J]. Geochimica et Cosmochimica Acta, 2003, 67(22): 4231−4250. doi: 10.1016/S0016-7037(03)00266-7 [21] 李津, 朱祥坤, 唐索寒. 低温环境下铁同位素分馏的若干重要过程[J]. 岩石矿物学杂志, 2008, 27(4): 305−316. doi: 10.3969/j.issn.1000-6524.2008.04.007Li Jin, Zhu Xiangkun, Tang Suohan. Fe isotope fractionation during low temperature process[J]. Acta Petrologica et Mineralogica, 2008, 27(4): 305−316. doi: 10.3969/j.issn.1000-6524.2008.04.007 [22] Hill P S, Schauble E A, Young E D. Effects of changing solution chemistry on Fe3+/Fe2+ isotope fractionation in aqueous Fe–Cl solutions[J]. Geochimica et Cosmochimica Acta, 2010, 74(23): 6669−6689. doi: 10.1016/j.gca.2010.08.038 [23] Beard B L, Johnson C M, Cox L, et al. Iron isotope biosignatures[J]. Science, 1999, 285(5435): 1889−1892. doi: 10.1126/science.285.5435.1889 [24] Dauphas N, Rouxel O. Mass spectrometry and natural variations of iron isotopes[J]. Mass Spectrometry Reviews, 2006, 25(4): 515−550. doi: 10.1002/mas.20078 [25] 何永胜, 胡东平, 朱传卫. 地球科学中铁同位素研究进展[J]. 地学前缘, 2015, 22(5): 54−71.He Yongsheng, Hu Dongping, Zhu Chuanwei. Progress of iron isotope geochemistry in geoscience[J]. Earth Science Frontiers, 2015, 22(5): 54−71. [26] Dauphas N, John S G, Rouxel O. Iron isotope systematics[J]. Reviews in Mineralogy and Geochemistry, 2017, 82(1): 415−510. doi: 10.2138/rmg.2017.82.11 [27] Teng Fangzhen, Dauphas N, Huang Shichun, et al. Iron isotopic systematics of oceanic basalts[J]. Geochimica et Cosmochimica Acta, 2013, 107: 12−26. doi: 10.1016/j.gca.2012.12.027 [28] Chen Shuo, Niu Yaoling, Guo Pengyuan, et al. Iron isotope fractionation during mid-ocean ridge basalt (MORB) evolution: evidence from lavas on the East Pacific Rise at 10°30′ N and its implications[J]. Geochimica et Cosmochimica Acta, 2019, 267: 227−239. doi: 10.1016/j.gca.2019.09.031 [29] Sun Pu, Niu Yaoling, Guo Pengyuan, et al. Large iron isotope variation in the eastern Pacific mantle as a consequence of ancient low-degree melt metasomatism[J]. Geochimica et Cosmochimica Acta, 2020, 286: 269−288. doi: 10.1016/j.gca.2020.07.029 [30] Gleeson M L M, Gibson S A, Williams H M. Novel insights from Fe-isotopes into the lithological heterogeneity of Ocean Island Basalts and plume-influenced MORBs[J]. Earth and Planetary Science Letters, 2020, 535: 116114. doi: 10.1016/j.jpgl.2020.116114 [31] Williams H M, Peslier A H, McCammon C, et al. Systematic iron isotope variations in mantle rocks and minerals: the effects of partial melting and oxygen fugacity[J]. Earth and Planetary Science Letters, 2005, 235(1/2): 435−452. [32] Weyer S, Ionov D A. Partial melting and melt percolation in the mantle: the message from Fe isotopes[J]. Earth and Planetary Science Letters, 2007, 259(1/2): 119−133. [33] Dauphas N, Craddock P R, Asimow P D, et al. Iron isotopes may reveal the redox conditions of mantle melting from Archean to Present[J]. Earth and Planetary Science Letters, 2009, 288(1/2): 255−267. [34] Dohmen R, Chakraborty S. Fe–Mg diffusion in olivine II: point defect chemistry, change of diffusion mechanisms and a model for calculation of diffusion coefficients in natural olivine[J]. Physics and Chemistry of Minerals, 2007, 34(6): 409−430. doi: 10.1007/s00269-007-0158-6 [35] Huang F, Chakraborty P, Lundstrom C C, et al. Isotope fractionation in silicate melts by thermal diffusion[J]. Nature, 2010, 464(7287): 396−400. doi: 10.1038/nature08840 [36] Teng Fangzhen, Dauphas N, Helz R T, et al. Diffusion-driven magnesium and iron isotope fractionation in Hawaiian olivine[J]. Earth and Planetary Science Letters, 2011, 308(3/4): 317−324. [37] Wu Hongjie, He Yongsheng, Teng Fangzhen, et al. Diffusion-driven magnesium and iron isotope fractionation at a gabbro-granite boundary[J]. Geochimica et Cosmochimica Acta, 2018, 222: 671−684. doi: 10.1016/j.gca.2017.11.010 [38] Lesher C E, Dannberg J, Barfod G H, et al. Iron isotope fractionation at the core-mantle boundary by thermodiffusion[J]. Nature Geoscience, 2020, 13(5): 382−386. doi: 10.1038/s41561-020-0560-y [39] Heimann A, Beard B L, Johnson C M. The role of volatile exsolution and sub-solidus fluid/rock interactions in producing high 56Fe/54Fe ratios in siliceous igneous rocks[J]. Geochimica et Cosmochimica Acta, 2008, 72(17): 4379−4396. doi: 10.1016/j.gca.2008.06.009 [40] Du Dehong, Wang Xiaolei, Yang Tao, et al. Origin of heavy Fe isotope compositions in high-silica igneous rocks: a rhyolite perspective[J]. Geochimica et Cosmochimica Acta, 2017, 218: 58−72. doi: 10.1016/j.gca.2017.09.014 [41] Du Dehong, Li Weiqiang, Wang Xiaolei, et al. Fe isotopic fractionation during the magmatic-hydrothermal stage of granitic magmatism[J]. Lithos, 2019, 350−351: 105265. doi: 10.1016/j.lithos.2019.105265 [42] Chen Liemeng, Song Xieyan, Zhu Xiangkun, et al. Iron isotope fractionation during crystallization and sub-solidus re-equilibration: constraints from the Baima mafic layered intrusion, SW China[J]. Chemical Geology, 2014, 380: 97−109. doi: 10.1016/j.chemgeo.2014.04.020 [43] Williams H M, Prytulak J, Woodhead J D, et al. Interplay of crystal fractionation, sulfide saturation and oxygen fugacity on the iron isotope composition of arc lavas: an example from the Marianas[J]. Geochimica et Cosmochimica Acta, 2018, 226: 224−243. doi: 10.1016/j.gca.2018.02.008 [44] Chen Yanhong, Niu Yaoling, Duan Meng, et al. Fractional crystallization causes the iron isotope contrast between mid-ocean ridge basalts and abyssal peridotites[J]. Communications Earth & Environment, 2021, 2(1): 1−9. [45] Richter M, Nebel O, Schwindinger M, et al. Competing effects of spreading rate, crystal fractionation and source variability on Fe isotope systematics in mid-ocean ridge lavas[J]. Scientific Reports, 2021, 11(1): 4123. doi: 10.1038/s41598-021-83387-7 [46] Rouxel O, Dobbek N, Ludden J, et al. Iron isotope fractionation during oceanic crust alteration[J]. Chemical Geology, 2003, 202(1/2): 155−182. [47] Berglund M, Wieser M E. Isotopic compositions of the elements 2009 (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2011, 83(2): 397−410. doi: 10.1351/PAC-REP-10-06-02 [48] 秦燕, 徐衍明, 侯可军, 等. 铁同位素分析测试技术研究进展[J]. 岩矿测试, 2020, 39(2): 151−161.Qin Yan, Xu Yanming, Hou Kejun, et al. Progress of analytical techniques for stable iron isotopes[J]. Rock and Mineral Analysis, 2020, 39(2): 151−161. [49] Dauphas N, Cook D L, Sacarabany A, et al. Iron 60 evidence for early injection and efficient mixing of stellar debris in the protosolar nebula[J]. The Astrophysical Journal, 2008, 686(1): 560−569. doi: 10.1086/589959 [50] Tang Haolan, Dauphas N. Abundance, distribution, and origin of 60Fe in the solar protoplanetary disk[J]. Earth and Planetary Science Letters, 2012, 359−360: 248−263. doi: 10.1016/j.jpgl.2012.10.011 [51] 朱祥坤, 李志红, 赵新苗, 等. 铁同位素的MC-ICP-MS测定方法与地质标准物质的铁同位素组成[J]. 岩石矿物学杂志, 2008, 27(4): 263−272. doi: 10.3969/j.issn.1000-6524.2008.04.001Zhu Xiangkun, Li Zhihong, Zhao Xinmiao, et al. High-precision measurements of Fe isotopes using MC-ICP-MS and Fe isotope compositions of geological reference materials[J]. Acta Petrologica et Mineralogica, 2008, 27(4): 263−272. doi: 10.3969/j.issn.1000-6524.2008.04.001 [52] Craddock P R, Dauphas N. Iron isotopic compositions of geological reference materials and chondrites[J]. Geostandards and Geoanalytical Research, 2011, 35(1): 101−123. doi: 10.1111/j.1751-908X.2010.00085.x [53] Beard B L, Johnson C M. High precision iron isotope measurements of terrestrial and lunar materials[J]. Geochimica et Cosmochimica Acta, 1999, 63(11/12): 1653−1660. [54] Williams H M, McCammon C A, Peslier A H, et al. Iron isotope fractionation and the oxygen fugacity of the mantle[J]. Science, 2004, 304(5677): 1656−1659. doi: 10.1126/science.1095679 [55] Teng Fangzhen, Dauphas N, Helz R T. Iron isotope fractionation during magmatic differentiation in Kilauea Iki lava lake[J]. Science, 2008, 320(5883): 1620−1622. doi: 10.1126/science.1157166 [56] Williams H M, Bizimis M. Iron isotope tracing of mantle heterogeneity within the source regions of oceanic basalts[J]. Earth and Planetary Science Letters, 2014, 404: 396−407. doi: 10.1016/j.jpgl.2014.07.033 [57] Craddock P R, Warren J M, Dauphas N. Abyssal peridotites reveal the near-chondritic Fe isotopic composition of the Earth[J]. Earth and Planetary Science Letters, 2013, 365: 63−76. doi: 10.1016/j.jpgl.2013.01.011 [58] Niu Yaoling, Wilson M, Humphreys E R, et al. A trace element perspective on the source of ocean island basalts (OIB) and fate of subducted ocean crust (SOC) and mantle lithosphere (SML)[J]. Episodes, 2012, 35(2): 310−327. doi: 10.18814/epiiugs/2012/v35i2/002 [59] Dauphas N, Roskosz M, Alp E E, et al. Magma redox and structural controls on iron isotope variations in Earth’s mantle and crust[J]. Earth and Planetary Science Letters, 2014, 398: 127−140. doi: 10.1016/j.jpgl.2014.04.033 [60] Dauphas N, Pourmand A, Teng Fangzhen. Routine isotopic analysis of iron by HR-MC-ICPMS: how precise and how accurate?[J]. Chemical Geology, 2009, 267(3/4): 175−184. [61] Polyakov V B, Mineev S D. The use of Mössbauer spectroscopy in stable isotope geochemistry[J]. Geochimica et Cosmochimica Acta, 2000, 64(5): 849−865. doi: 10.1016/S0016-7037(99)00329-4 [62] Polyakov V B, Clayton R N, Horita J, et al. Equilibrium iron isotope fractionation factors of minerals: reevaluation from the data of nuclear inelastic resonant X-ray scattering and Mössbauer spectroscopy[J]. Geochimica et Cosmochimica Acta, 2007, 71(15): 3833−3846. doi: 10.1016/j.gca.2007.05.019 [63] Schuessler J A, Schoenberg R, Behrens H, et al. The experimental calibration of the iron isotope fractionation factor between pyrrhotite and peralkaline rhyolitic melt[J]. Geochimica et Cosmochimica Acta, 2007, 71(2): 417−433. doi: 10.1016/j.gca.2006.09.012 [64] Schoenberg R, Marks M A W, Schuessler J A, et al. Fe isotope systematics of coexisting amphibole and pyroxene in the alkaline igneous rock suite of the Ilímaussaq Complex, South Greenland[J]. Chemical Geology, 2009, 258(1/2): 65−77. [65] Canil D, O'Neill H S C, Pearson D G, et al. Ferric iron in peridotites and mantle oxidation states[J]. Earth and Planetary Science Letters, 1994, 123(1/3): 205−220. [66] He Yongsheng, Meng Xunan, Ke Shan, et al. A nephelinitic component with unusual δ56Fe in Cenozoic basalts from eastern China and its implications for deep oxygen cycle[J]. Earth and Planetary Science Letters, 2019, 512: 175−183. doi: 10.1016/j.jpgl.2019.02.009 [67] Sossi P A, O’Neill H S C. The effect of bonding environment on iron isotope fractionation between minerals at high temperature[J]. Geochimica et Cosmochimica Acta, 2017, 196: 121−143. doi: 10.1016/j.gca.2016.09.017 [68] Zhao Xinmiao, Zhang Hongfu, Zhu Xiangkun, et al. Iron isotope variations in spinel peridotite xenoliths from North China Craton: implications for mantle metasomatism[J]. Contributions to Mineralogy and Petrology, 2010, 160(1): 1−14. doi: 10.1007/s00410-009-0461-y [69] Beard B L, Johnson C M. Inter-mineral Fe isotope variations in mantle-derived rocks and implications for the Fe geochemical cycle[J]. Geochimica et Cosmochimica Acta, 2004, 68(22): 4727−4743. doi: 10.1016/j.gca.2004.04.023 [70] Williams H M, Nielsen S G, Renac C, et al. Fractionation of oxygen and iron isotopes by partial melting processes: implications for the interpretation of stable isotope signatures in mafic rocks[J]. Earth and Planetary Science Letters, 2009, 283(1/4): 156−166. [71] Zhao Xinmiao, Cao Huihui, Mi Xue, et al. Combined iron and magnesium isotope geochemistry of pyroxenite xenoliths from Hannuoba, North China Craton: implications for mantle metasomatism[J]. Contributions to Mineralogy and Petrology, 2017, 172(6): 1−26. doi: 10.1007/s00410-017-1356-y [72] Nebel O, Sossi P A, Bénard A, et al. Reconciling petrological and isotopic mixing mechanisms in the Pitcairn mantle plume using stable Fe isotopes[J]. Earth and Planetary Science Letters, 2019, 521: 60−67. doi: 10.1016/j.jpgl.2019.05.037 [73] Konter J G, Pietruszka A J, Hanan B B, et al. Unusual δ56Fe values in Samoan rejuvenated lavas generated in the mantle[J]. Earth and Planetary Science Letters, 2016, 450: 221−232. doi: 10.1016/j.jpgl.2016.06.029 [74] Poitrasson F, Halliday A N, Lee D C, et al. Iron isotope differences between Earth, Moon, Mars and Vesta as possible records of contrasted accretion mechanisms[J]. Earth and Planetary Science Letters, 2004, 223(3/4): 253−266. [75] Weyer S, Anbar A D, Brey G P, et al. Iron isotope fractionation during planetary differentiation[J]. Earth and Planetary Science Letters, 2005, 240(2): 251−264. doi: 10.1016/j.jpgl.2005.09.023 [76] Poitrasson F, Freydier R. Heavy iron isotope composition of granites determined by high resolution MC-ICP-MS[J]. Chemical Geology, 2005, 222(1/2): 132−147. [77] Telus M, Dauphas N, Moynier F, et al. Iron, zinc, magnesium and uranium isotopic fractionation during continental crust differentiation: the tale from migmatites, granitoids, and pegmatites[J]. Geochimica et Cosmochimica Acta, 2012, 97: 247−265. doi: 10.1016/j.gca.2012.08.024 [78] Simon A C, Pettke T, Candela P A, et al. Magnetite solubility and iron transport in magmatic-hydrothermal environments[J]. Geochimica et Cosmochimica Acta, 2004, 68(23): 4905−4914. doi: 10.1016/j.gca.2004.05.033 [79] Sossi P A, Foden J D, Halverson G P. Redox-controlled iron isotope fractionation during magmatic differentiation: an example from the Red Hill intrusion, S. Tasmania[J]. Contributions to Mineralogy and Petrology, 2012, 164(5): 757−772. doi: 10.1007/s00410-012-0769-x [80] Schuessler J A, Schoenberg R, Sigmarsson O. Iron and lithium isotope systematics of the Hekla volcano, Iceland—evidence for Fe isotope fractionation during magma differentiation[J]. Chemical Geology, 2009, 258(1/2): 78−91. [81] Richter F M, Watson E B, Mendybaev R, et al. Isotopic fractionation of the major elements of molten basalt by chemical and thermal diffusion[J]. Geochimica et Cosmochimica Acta, 2009, 73(14): 4250−4263. doi: 10.1016/j.gca.2009.04.011 [82] Lundstrom C. Hypothesis for the origin of convergent margin granitoids and Earth’s continental crust by thermal migration zone refining[J]. Geochimica et Cosmochimica Acta, 2009, 73(19): 5709−5729. doi: 10.1016/j.gca.2009.06.020 [83] Zambardi T, Lundstrom C C, Li Xiaoxiao, et al. Fe and Si isotope variations at Cedar Butte volcano; insight into magmatic differentiation[J]. Earth and Planetary Science Letters, 2014, 405: 169−179. doi: 10.1016/j.jpgl.2014.08.020 [84] Mills R, Harris K R. The effect of isotopic substitution on diffusion in liquids[J]. Chemical Society Reviews, 1976, 5(2): 215−231. [85] Foden J, Sossi P A, Wawryk C M. Fe isotopes and the contrasting petrogenesis of A-, I- and S-type granite[J]. Lithos, 2015, 212−215: 32−44. doi: 10.1016/j.lithos.2014.10.015 [86] Roskosz M, Sio C K I, Dauphas N, et al. Spinel-olivine-pyroxene equilibrium iron isotopic fractionation and applications to natural peridotites[J]. Geochimica et Cosmochimica Acta, 2015, 169: 184−199. doi: 10.1016/j.gca.2015.07.035 [87] Staudigel H, Hart S R. Alteration of basaltic glass: mechanisms and significance for the oceanic crust-seawater budget[J]. Geochimica et Cosmochimica Acta, 1983, 47(3): 337−350. doi: 10.1016/0016-7037(83)90257-0 [88] Staudigel H, Plank T, White B, et al. Geochemical fluxes during seafloor alteration of the basaltic upper oceanic crust: DSDP Sites 417 and 418[J]. Subduction: Top to Bottom, 1996, 96: 19−38. [89] Alt J C, Teagle D A H. The uptake of carbon during alteration of ocean crust[J]. Geochimica et Cosmochimica Acta, 1999, 63(10): 1527−1535. doi: 10.1016/S0016-7037(99)00123-4 [90] Wheat C G, Mottl M J. Composition of pore and spring waters from Baby Bare: global implications of geochemical fluxes from a ridge flank hydrothermal system[J]. Geochimica et Cosmochimica Acta, 2000, 64(4): 629−642. doi: 10.1016/S0016-7037(99)00347-6 [91] Alt J C. Subseafloor processes in mid-ocean ridge hydrothermal systems[M]//Humphris S E, Zierenberg R A, Mullineaux L S, et al. Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geological Interactions, Volume 91. Washington: American Geophysical Union, 1995: 85−114. [92] Honnorez J. The aging of the oceanic crust at low temperature[J]. The Oceanic Lithosphere, 1981: 525−587. [93] Liu Yang, Spicuzza M J, Craddock P R, et al. Oxygen and iron isotope constraints on near-surface fractionation effects and the composition of lunar mare basalt source regions[J]. Geochimica et Cosmochimica Acta, 2010, 74(21): 6249−6262. doi: 10.1016/j.gca.2010.08.008 [94] Polyakov V B. Equilibrium iron isotope fractionation at core-mantle boundary conditions[J]. Science, 2009, 323(5916): 912−914. doi: 10.1126/science.1166329 [95] Williams H M, Wood B J, Wade J, et al. Isotopic evidence for internal oxidation of the Earth’s mantle during accretion[J]. Earth and Planetary Science Letters, 2012, 321−322: 54−63. doi: 10.1016/j.jpgl.2011.12.030 [96] Weyer S, Seitz H M. Coupled lithium- and iron isotope fractionation during magmatic differentiation[J]. Chemical Geology, 2012, 294−295: 42−50. doi: 10.1016/j.chemgeo.2011.11.020 [97] Dauphas N, Teng Fangzhen, Arndt N T. Magnesium and iron isotopes in 2.7 Ga Alexo komatiites: mantle signatures, no evidence for Soret diffusion, and identification of diffusive transport in zoned olivine[J]. Geochimica et Cosmochimica Acta, 2010, 74(11): 3274−3291. doi: 10.1016/j.gca.2010.02.031 [98] Zhao Xinmiao, Zhang Hongfu, Zhu Xiangkun, et al. Iron isotope evidence for multistage melt-peridotite interactions in the lithospheric mantle of eastern China[J]. Chemical Geology, 2012, 292−293: 127−139. doi: 10.1016/j.chemgeo.2011.11.016 [99] Huang Fang, Zhang Zhaofeng, Lundstrom C C, et al. Iron and magnesium isotopic compositions of peridotite xenoliths from eastern China[J]. Geochimica et Cosmochimica Acta, 2011, 75(12): 3318−3334. doi: 10.1016/j.gca.2011.03.036 [100] Hibbert K E J, Williams H M, Kerr A C, et al. Iron isotopes in ancient and modern komatiites: evidence in support of an oxidised mantle from Archean to present[J]. Earth and Planetary Science Letters, 2012, 321−322: 198−207. doi: 10.1016/j.jpgl.2012.01.011 [101] Poitrasson F, Delpech G, Grégoire M. On the iron isotope heterogeneity of lithospheric mantle xenoliths: implications for mantle metasomatism, the origin of basalts and the iron isotope composition of the Earth[J]. Contributions to Mineralogy and Petrology, 2013, 165(6): 1243−1258. doi: 10.1007/s00410-013-0856-7 [102] Beard B L, Johnson C M, Skulan J L, et al. Application of Fe isotopes to tracing the geochemical and biological cycling of Fe[J]. Chemical Geology, 2003, 195(1/4): 87−117. [103] Peters B J, Shahar A, Carlson R W, et al. A sulfide perspective on iron isotope fractionation during ocean island basalt petrogenesis[J]. Geochimica et Cosmochimica Acta, 2019, 245: 59−78. doi: 10.1016/j.gca.2018.10.015 [104] Nebel O, Sossi P A, Bénard A, et al. Redox-variability and controls in subduction zones from an iron-isotope perspective[J]. Earth and Planetary Science Letters, 2015, 432: 142−151. doi: 10.1016/j.jpgl.2015.09.036 [105] Nebel O, Sossi P A, Foden J, et al. Iron isotope variability in ocean floor lavas and mantle sources in the Lau back-arc basin[J]. Geochimica et Cosmochimica Acta, 2018, 241: 150−163. doi: 10.1016/j.gca.2018.08.046 -



 下载:
下载: