Effects of light and temperature on the photosynthetic pathway and antioxidant function of two green tide species
-
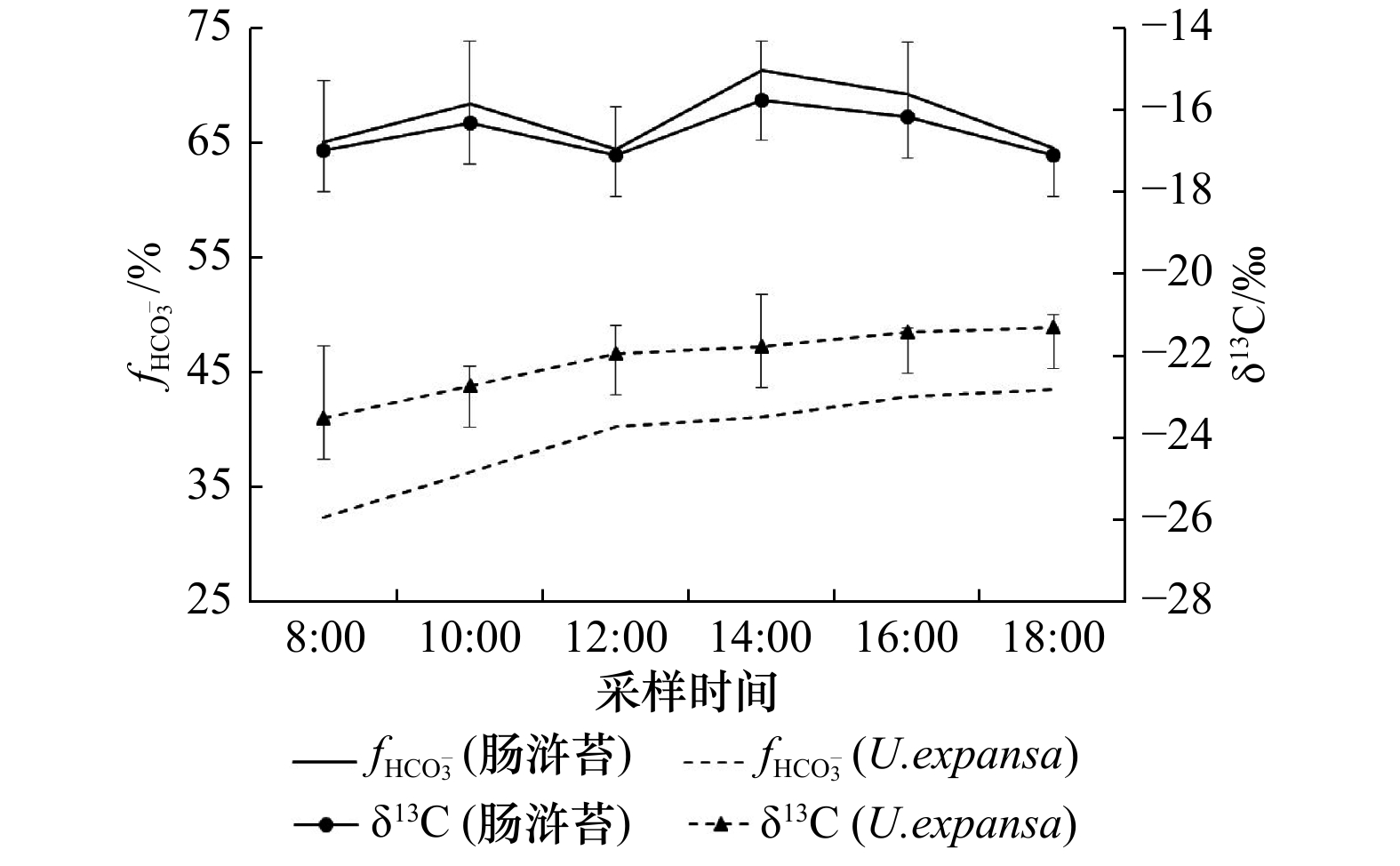
摘要: 绿潮是潮间带绿藻大量增殖形成的高生物量生态灾害,其暴发不仅受到温度、营养盐等环境因素的驱动,而且与自身光合能力的强弱密切相关。本研究以绿潮物种—肠浒苔(Ulva intestinalis)和Ulva expansa为研究对象,通过室外培养实验,检测了它们在夏季高温、高光强条件下的光合途径与抗氧化生理特征,并分析了与光合产物的对应关系。研究结果表明,肠浒苔与U. expansa的光合途径与抗氧化能力存在显著差异。前者的C4光合途径关键酶活性在光合作用过程中出现高表达特征,与光、温度存在显著相关性,C3光合途径关键酶活性在中午受到强光抑制;组织δ13C的变化范围为−17.1‰~−15.7‰,表明其光合作用可能由C3和C4途径共同参与。后者的C4光合途径关键酶活性表达较弱,且与光、温度不存在显著相关性,C3光合途径关键酶活性没有出现明显的光抑制现象;组织δ13C的范围为−23.5‰~−21.9‰,表明其光合作用主要依靠C3途径进行。此外,肠浒苔在培养过程中表现出了较强的抗氧化能力,可能与其在高温、高光强条件下启动C4光合途径密切相关。肠浒苔与U. expansa的比较研究说明,藻类C4光合途径存在显著种间差异性。
-
关键词:
- 肠浒苔 /
- Ulva expansa /
- 卡尔文循环 /
- Hatch-Slack光合途径 /
- 光合固碳
Abstract: Green tide is a high biomass ecological disaster caused by the proliferation of green algae in intertidal zone. Its outbreak is not only driven by environmental factors such as temperature and nutrients, but also closely related to its own photosynthetic capacity. In this study, Ulva intestinalis and Ulva expansa, the two green tide species, were selected for the outdoor culture experiment. The photosynthetic pathway, antioxidant physiological characteristics and their corresponding relationship with photosynthetic products of the two species were detected and compared under the conditions of high temperature and high light intensity in summer. The results showed that there were significant differences in photosynthetic pathway and antioxidant capacity between U. intestinalis and U. expansa. The key enzyme activity of C4 photosynthetic pathway was highly expressed in the process of photosynthesis of the former, which was significantly correlated with light and temperature. The key enzyme activity of C3 photosynthetic pathway of U. intestinalis was inhibited by strong light at noon, and the change range of δ13C in tissue ranged from −17.1‰ to −15.7‰, which indicated that C3 and C4 pathway might be involved in its photosynthetic cooperation. For U. expansa, the expression of key enzymes in C4 photosynthetic pathway was weak and there was no significant correlation with light and temperature. Also, there was no obvious photoinhibition in C3 photosynthetic pathway and the range of δ13C in tissue is from −23.5‰ to −21.9‰, indicating that the photosynthesis mainly depends on C3 pathway. In addition, U. intestinalis showed a strong antioxidant capacity in the process of culture, which may be closely related to its C4 photosynthetic pathway under high temperature and high light conditions. The comparative study between U. intestinalis and U. expansa showed that there were significant differences in the initiation of C4 photosynthetic pathway between algal species, and the comparative study on the photosynthetic mechanism of different green algal species needs further exploration. -
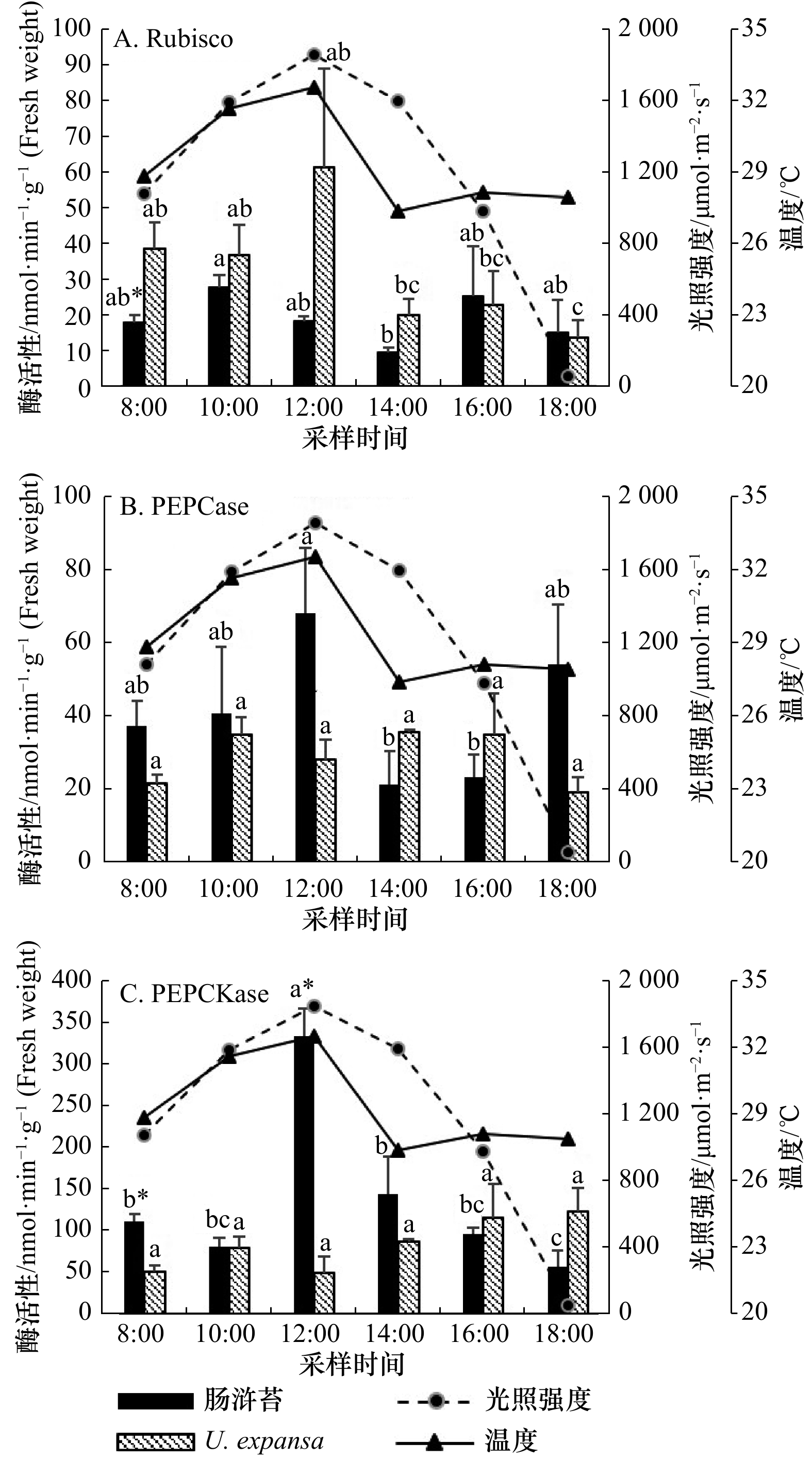
图 2 两种绿藻C3关键酶(Rubisco)与C4关键酶(PEPCase、PEPCKase)活性的日变化特征比较
*表示两个物种之间酶活性具有显著性差异,误差棒上的不同小写字母(a, b, c)表示同一物种在不同时间下酶活性有显著性差异(p<0.05)
Fig. 2 The comparison of diurnal variations of C3 key enzyme (Rubisco) and C4 key enzyme (PEPCase and PEPCKase) activities between U. intestinalis and U. expansa
*represents there is a significant difference in enzyme activity between the two species. Different lowercase letters (a, b, c) on the error bars indicate that the same species has a significant difference (p<0.05) in enzyme activity at different times
图 4 两种绿藻抗氧化物酶(SOD、POD)活性和MDA含量的日变化特征比较
*表示两个物种之间酶活性具有显著性差异,误差棒上的不同小写字母(a, b, c)表示同一物种在不同时间下酶活性有显著性差异(p<0.05)
Fig. 4 The comparison of antioxidant enzymes (SOD, POD) actirities and MDA content between U. intestinalis and U. expansa, corresponding to diurnal variations
*represents there is a significant difference in enzyme activity between the two species. Different lowercase letters (a, b, c) on the error bars indicate that the same species has a significant difference (p<0.05) in enzyme activity at different times
表 1 两种绿藻光合作用酶活性与温度、光强的相关性
Tab. 1 Correlations between photosynthetic enzyme activities of U. intestinalis and U. expansa vs. temperature and light intensity
参数 肠浒苔 U. expansa 温度 光强 温度 光强 Rubisco 0.080 0.118 0.700** 0.396 PEPCase 0.429 −0.048 0.068 0.458 PEPCKase 0.688** 0.590* −0.648** −0.515* 注:**表示在0.01水平(双侧)上显著相关;*表示在0.05水平(双侧)上显著相关。 表 2 两种绿藻的抗氧化酶(SOD,POD)活性以及MDA含量与温度、光强的相关性
Tab. 2 Correlation between antioxidase activities, MDA content of U. intestinalis and U. expansa vs. temperature and light intensity
参数 肠浒苔 U. expansa 温度 光强 温度 光强 SOD 0.578* 0.825** 0.277 0.432 POD 0.171 −0.210 0.296 0.368 MDA 0.561* 0.627** 0.390 0.145 注:**表示在0.01水平(双侧)上显著相关;*表示在0.05水平(双侧)上显著相关。 -
[1] Fletcher R L. The occurrence of “green tides” — a review[M]//Schramm W, Nienhuis P H. Marine Benthic Vegetation. Berlin: Springer, 1996: 7−43. [2] Blomster J, Bäck S, Fewer D P, et al. Novel morphology in Enteromorpha (Ulvophyceae) forming green tides[J]. American Journal of Botany, 2002, 89(11): 1756−1763. doi: 10.3732/ajb.89.11.1756 [3] Bäck S, Lehvo A, Blomster J. Mass occurrence of unattached Enteromorpha intestinalis on the Finnish Baltic Sea coast[J]. Annales Botanici Fennici, 2000, 37: 155−161. [4] Cohen R A, Fong P. Physiological responses of a bloom-forming green macroalga to short-term change in salinity, nutrients, and light help explain its ecological success[J]. Estuaries, 2004, 27(2): 209−216. doi: 10.1007/BF02803378 [5] Kamer K, Boyle K A, Fong P. Macroalgal bloom dynamics in a highly eutrophic southern California estuary[J]. Estuaries, 2001, 24(4): 623−635. doi: 10.2307/1353262 [6] Liu Dongyan, Keesing J K, Xing Qianguo, et al. World’s largest macroalgal bloom caused by expansion of seaweed aquaculture in China[J]. Marine Pollution Bulletin, 2009, 58(6): 888−895. doi: 10.1016/j.marpolbul.2009.01.013 [7] Wang Chao, Yu Rencheng, Zhou Mingjiang. Effects of the decomposing green macroalga Ulva (Enteromorpha) prolifera on the growth of four red-tide species[J]. Harmful Algae, 2012, 16: 12−19. doi: 10.1016/j.hal.2011.12.007 [8] Rosenberg C, Ramus J. Ecological growth strategies in the seaweeds Gracilaria foliifera (Rhodophyceae) and Ulva sp. (Chlorophyceae): soluble nitrogen and reserve carbohydrates[J]. Marine Biology, 1982, 66(3): 251−259. doi: 10.1007/BF00397030 [9] Kim J H, Kang E J, Park M G, et al. Effects of temperature and irradiance on photosynthesis and growth of a green-tide-forming species (Ulva linza) in the Yellow Sea[J]. Journal of Applied Phycology, 2011, 23(3): 421−432. doi: 10.1007/s10811-010-9590-y [10] Bassham J A. Kinetic studies of the photosynthetic carbon reduction cycle[J]. Annual Review of Plant Physiology, 1964, 15(1): 101−120. doi: 10.1146/annurev.pp.15.060164.000533 [11] Raven J A. Carbon dioxide fixation[M]//Stewart W D P. Algal Physiology and Biochemistry. Berkeley: University of California Press, 1974: 434−455. [12] Cassar N, Laws E A. Potential contribution of β-carboxylases to photosynthetic carbon isotope fractionation in a marine diatom[J]. Phycologia, 2007, 46(3): 307−314. doi: 10.2216/06-50.1 [13] Karekar M D, Joshi G V. Photosynthetic carbon metabolism in marine algae[J]. Botanica Marina, 1973, 16(4): 216−220. [14] Reinfelder J R, Kraepiel A M L, Morel F M M. Unicellular C4 photosynthesis in a marine diatom[J]. Nature, 2000, 407(6807): 996−999. doi: 10.1038/35039612 [15] Xu Jianfang, Fan Xiao, Zhang Xiaowen, et al. Evidence of coexistence of C3 and C4 photosynthetic pathways in a green-tide-forming alga, Ulva prolifera[J]. PLoS One, 2012, 7(5): e37438. doi: 10.1371/journal.pone.0037438 [16] Valiela I, Liu Dongyan, Lloret J, et al. Stable isotopic evidence of nitrogen sources and C4 metabolism driving the world's largest macroalgal green tides in the Yellow Sea[J]. Scientific Reports, 2018, 8(1): 17437. doi: 10.1038/s41598-018-35309-3 [17] Reiskind J B, Beer S, Bowes G. Photosynthesis, photorespiration and ecophysiological interactions in marine macroalgae[J]. Aquatic Botany, 1989, 34(1/3): 131−152. [18] Cooper J P. Potential production and energy conversion in temperate and tropical grasses[J]. Herbage Abstracts, 1970, 40: 1−13. [19] Cooper J P, Tainton N M. Light and temperature requirements for the growth of tropical and temperate grasses[J]. Herbage Abstract, 1968, 38(3): 167−177. [20] Joshi M C, Boyer J S, Kramer P J. Growth, carbon dioxide exchange, transpiration, and transpiration ratio of pineapple[J]. Botanical Gazette, 1965, 126(3): 174−179. doi: 10.1086/336315 [21] Black C C Jr. Photosynthetic carbon fixation in relation to net CO2 uptake[J]. Annual Review of Plant Physiology, 1973, 24(1): 253−286. doi: 10.1146/annurev.pp.24.060173.001345 [22] Bjorkman O, Boardman N K, Anderson J M, et al. Effect of light intensity during growth of Atriplex patula on the capacity of photosynthetic reactions, chloroplast components and structure[J]. Yearbook Carnegie Institution Washington, 1972, 71: 115−135. [23] Scandalios J G. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses[J]. Brazilian Journal of Medical and Biological Research, 2005, 38(7): 995−1014. doi: 10.1590/S0100-879X2005000700003 [24] Uzilday B, Turkan I, Ozgur R, et al. Strategies of ROS regulation and antioxidant defense during transition from C3 to C4 photosynthesis in the genus Flaveria under PEG-induced osmotic stress[J]. Journal of Plant Physiology, 2014, 171(1): 65−75. doi: 10.1016/j.jplph.2013.06.016 [25] Hughey J R, Miller K A, Gabrielson P W. Mitogenome analysis of a green tide forming Ulva from California, USA confirms its identity as Ulva expansa (Ulvaceae, Chlorophyta)[J]. Mitochondrial DNA Part B, 2018, 3(2): 1302−1303. doi: 10.1080/23802359.2018.1535859 [26] Shultz D J, Calder J A. Organic carbon 13C/12C variations in estuarine sediments[J]. Geochimica et Cosmochimica Acta, 1976, 40(4): 381−385. doi: 10.1016/0016-7037(76)90002-8 [27] Cornwall C E, Revill A T, Hall-Spencer J M, et al. Inorganic carbon physiology underpins macroalgal responses to elevated CO2[J]. Scientific Reports, 2017, 7: 46297. doi: 10.1038/srep46297 [28] Tiunov A V. Stable isotopes of carbon and nitrogen in soil ecological studies[J]. Biology Bulletin, 2007, 34(4): 395−407. doi: 10.1134/S1062359007040127 [29] Faganeli J, Vukovič A, Saleh F I, et al. C: N: P ratios and stable carbon and hydrogen isotopes in the benthic marine algae, Ulva rigida C. Ag. and Fucusvirsoides J. Ag[J]. Journal of Experimental Marine Biology and Ecology, 1986, 102(2/3): 153−166. [30] Guy R D, Vanlerberghe G C, Turpin D H. Significance of phosphoenolpyruvate carboxylase during ammonium assimilation: carbon isotope discrimination in photosynthesis and respiration by the N-limited green alga Selenastrum minutum[J]. Plant Physiology, 1989, 89(4): 1150−1157. doi: 10.1104/pp.89.4.1150 [31] Sültemeyer D. Carbonic anhydrase in eukaryotic algae: characterization, regulation, and possible function during photosynthesis[J]. Canadian Journal of Botany, 1998, 76(6): 962−972. doi: 10.1139/b98-082 [32] Badger M R, Andrews T J, Whitney S M, et al. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae[J]. Canadian Journal of Botany, 1998, 76(6): 1052−1071. doi: 10.1139/b98-074 [33] McGinn P J, Morel F M M. Expression and inhibition of the carboxylating and decarboxylating enzymes in the photosynthetic C4 pathway of marine diatoms[J]. Plant Physiology, 2008, 146(1): 300−309. doi: 10.1104/pp.107.110569 [34] Johnston A M, Raven J A, Beardall J, et al. Photosynthesis in a marine diatom[J]. Nature, 2001, 412(6842): 40−41. [35] Granum E, Raven J A, Leegood R C. How do marine diatoms fix 10 billion tonnes of inorganic carbon per year?[J]. Canadian Journal of Botany, 2005, 83(7): 898−908. doi: 10.1139/b05-077 [36] Haimovich-Dayan M, Garfinkel N, Ewe D, et al. The role of C4 metabolism in the marine diatom Phaeodactylum tricornutum[J]. New Phytologist, 2013, 197(1): 177−185. doi: 10.1111/j.1469-8137.2012.04375.x [37] Gowik U, Westhoff P. The path from C3 to C4 photosynthesis[J]. Plant Physiology, 2011, 155(1): 56−63. doi: 10.1104/pp.110.165308 [38] Roberts K, Granum E, Leegood R C, et al. C3 and C4 pathways of photosynthetic carbon assimilation in marine diatoms are under genetic, not environmental, control[J]. Plant Physiology, 2007, 145(1): 230−235. doi: 10.1104/pp.107.102616 [39] Rao S, Reiskind J, Bowes G. Light regulation of the photosynthetic phosphoenolpyruvate carboxylase (PEPC) in Hydrilla verticillata[J]. Plant and Cell Physiology, 2006, 47(9): 1206−1216. doi: 10.1093/pcp/pcj091 [40] Edwards G E, Nakamoto H, Burnell J N, et al. Pyruvate, Pi Dikinase and NADP-malate dehydrogenase in C4 photosynthesis: properties and mechanism of light/dark regulation[J]. Annual Review of Plant Physiology, 1985, 36(1): 255−286. doi: 10.1146/annurev.pp.36.060185.001351 [41] Moss D N, Musgrave R B, Lemon E R. Photosynthesis under field conditions. III. Some effects of light, carbon dioxide, temperature, and soil moisture on photosynthesis, respiration, and transpiration of corn[J]. Crop Science, 1961, 1(2): 83−87. doi: 10.2135/cropsci1961.0011183X000100020001x [42] Kalt-Torres W, Kerr P S, Usuda H, et al. Diurnal changes in maize leaf photosynthesis[J]. Plant Physiology, 1987, 83(2): 283−288. doi: 10.1104/pp.83.2.283 [43] 姜振升, 刘培培, 王美玲, 等. 黄瓜幼苗Rubisco与Rubisco活化酶对光强的响应[J]. 西北农业学报, 2011, 20(9): 95−99. doi: 10.3969/j.issn.1004-1389.2011.09.019Jiang Zhensheng, Liu Peipei, Wang Meiling, et al. Response of Rubisco and Rubisco activase in cucumber seedlings to light intensity[J]. Acta Agriculturae Boreali-Occidentalis Sinica, 2011, 20(9): 95−99. doi: 10.3969/j.issn.1004-1389.2011.09.019 [44] 翁晓燕, 陆庆, 蒋德安. 水稻Rubisco活化酶在调节Rubisco活性和光合日变化中的作用[J]. 中国水稻科学, 2001, 15(1): 35−40. doi: 10.3321/j.issn:1001-7216.2001.01.007Weng Xiaoyan, Lu Qing, Jiang Dean. Rubisco activase and its regulation on diurnal changes of photosynthetic rate and the activity of Ribulose-1, 5-bisphosphate carboxyase/oxygenase (Rubisco)[J]. Chinese Journal of Rice Science, 2001, 15(1): 35−40. doi: 10.3321/j.issn:1001-7216.2001.01.007 [45] Muraoka H, Tang Yanhong, Terashima I, et al. Contributions of diffusional limitation, photoinhibition and photorespiration to midday depression of photosynthesis in Arisaema heterophyllum in natural high light[J]. Plant, Cell and Environment, 2000, 23(3): 235−250. doi: 10.1046/j.1365-3040.2000.00547.x [46] Demmig-Adams B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves[J]. Plant and Cell Physiology, 1998, 39(5): 474−482. doi: 10.1093/oxfordjournals.pcp.a029394 [47] 黄雪清, 焦德茂. 转C4光合酶基因水稻株系的抗光氧化特性[J]. 植物生理学报, 2001, 27(5): 393−400. doi: 10.3321/j.issn:1671-3877.2001.05.006Huang Xueqing, Jiao Demao. The characteristics of resistance to photooxidation of transgenic rice (Oryza sativa L.) plants with maize genes coding for C4 photosynthesis enzyme[J]. Acta Phytophysiologica Sinica, 2001, 27(5): 393−400. doi: 10.3321/j.issn:1671-3877.2001.05.006 [48] 李霞, 焦德茂, 戴传超. 转PEPC基因水稻对光氧化逆境的响应[J]. 作物学报, 2005, 31(4): 408−413. doi: 10.3321/j.issn:0496-3490.2005.04.002Li Xia, Jiao Demao, Dai Chuanchao. The response to photooxidation in leaves of PEPC transgenic rice plant (Oryza sativa L.)[J]. Acta Agronomica Sinica, 2005, 31(4): 408−413. doi: 10.3321/j.issn:0496-3490.2005.04.002 [49] 周宝元. PEPC对水稻抗旱性的调节效果及其机理研究[D]. 北京: 中国农业科学院, 2011.Zhou Baoyuan. Effect of PEPC on rice drought resistance[D]. Beijing: Chinese Academy of Agricultural Sciences, 2011. [50] Casati P, Lara M V, Andreo C S. Induction of a C4-like mechanism of CO2 fixation in Egeria densa, a submersed aquatic species[J]. Plant Physiology, 2000, 123(4): 1611−1622. doi: 10.1104/pp.123.4.1611 [51] Rao S K, Magnin N C, Reiskind J B, et al. Photosynthetic and other phosphoenolpyruvate carboxylase isoforms in the single-cell, facultative C4 system of Hydrilla verticillata[J]. Plant Physiology, 2002, 130(2): 876−886. doi: 10.1104/pp.008045 [52] 张忠梁. 不同作物光合特性比较及籽粒苋C4途径关键酶基因的原核表达[D]. 太原: 山西大学, 2013.Zhang Zhongliang. Study on photosynthetic characteristics of different plants and prokaryotic expression of C4 key enzyme genes in Amaranthus hypochondriacus L.[D]. Taiyuan: Shanxi University, 2013. [53] 王超, 李霞, 蔡庆生. 不同测定环境条件下转PEPC基因水稻及杂交后代光合特性的比较[J]. 江苏农业学报, 2008, 24(3): 232−236. doi: 10.3969/j.issn.1000-4440.2008.03.003Wang Chao, Li Xia, Cai Qingsheng. Comparison of photosynthetic characteristics of PEPC transgenic rice and its hybrid rice lines under field and lab conditions[J]. Jiangsu Journal of Agricultural Sciences, 2008, 24(3): 232−236. doi: 10.3969/j.issn.1000-4440.2008.03.003 -




 下载:
下载: