The role of arachidonic acid regulatory network in the metabolism of paralytic shellfish toxins in Mytilus galloprovincialis: based on combined transcriptome and metabolome analysis
-
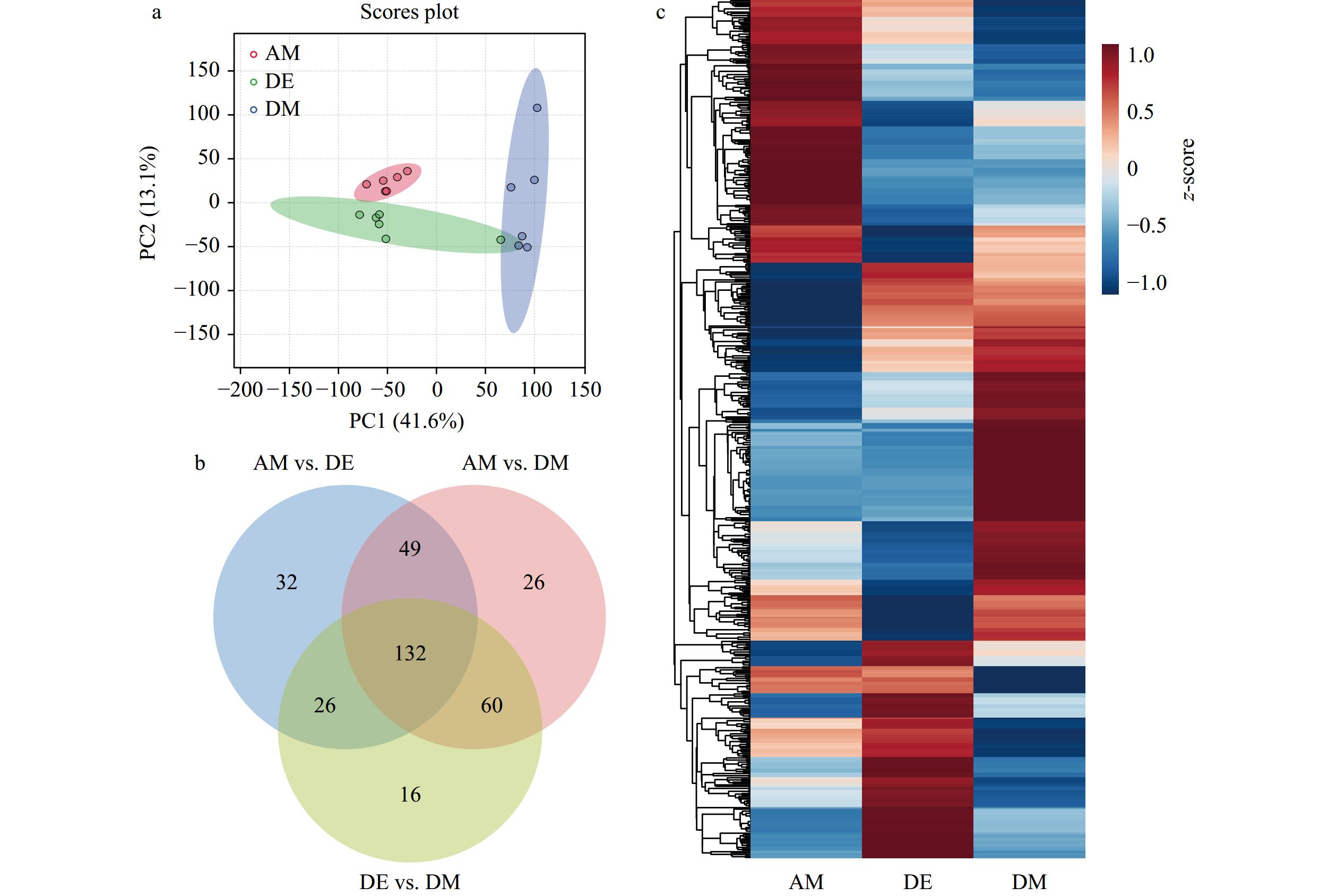
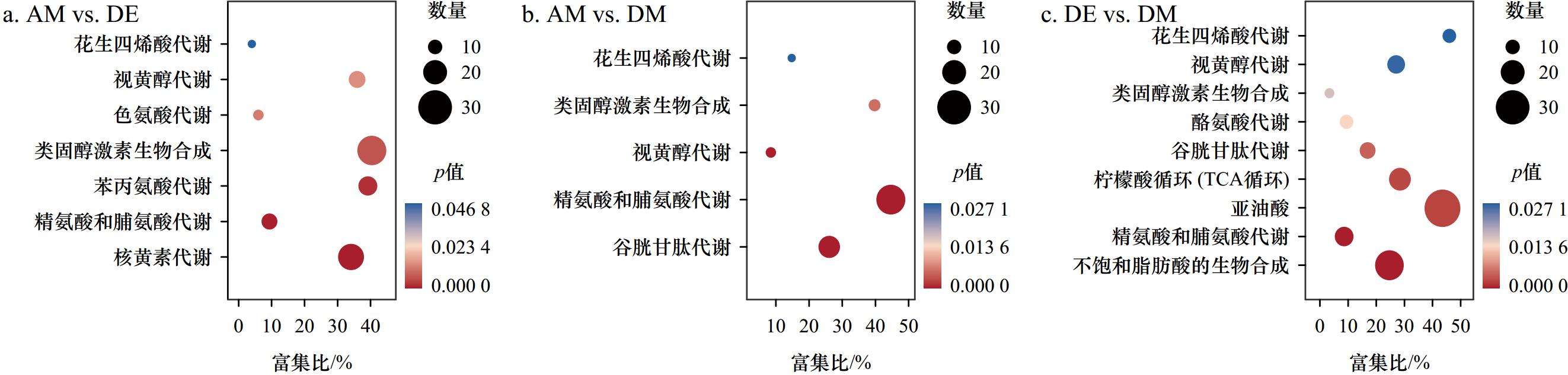
摘要: 紫贻贝(Mytilus galloprovincialis)是富集麻痹性贝类毒素(Paralytic Shellfish Toxins, PSTs)能力很强的代表性双壳贝类,但研究表明紫贻贝暴露于PSTs也会引起机体炎症反应,其作用机理及对毒素代谢影响尚不清楚。本研究采用转录组学与代谢组学联合分析技术,比较了产PSTs的链状亚历山大藻(Alexandrium catenella)暴发的不同时期,紫贻贝机体的基因表达量与代谢物含量差异,以解析PSTs胁迫下紫贻贝机体的炎症反应机制。结果表明,紫贻贝暴露于PSTs后表达的基因和代谢物均发生显著变化,其中差异表达基因17232个,差异代谢物341个。基于联合分析,差异表达基因与差异代谢物显著富集在花生四烯酸和谷胱甘肽代谢通路中,基因PLA2G2F、ALOX5、TBXAS1以及代谢物ARA、PGH2、TXA2、LTA4、5-HETE对贻贝机体的促炎反应发挥重要作用;而基因GPX4、CYP2J2和代谢物15-HETE、GSH则调节机体炎症的消退。本研究揭示花生四烯酸相关通路在贻贝机体炎症反应过程中具有重要的调控作用,为下一步深度揭示PSTs胁迫下贻贝机体炎症网络化响应机制提供了研究基础。Abstract: Mytilus galloprovincialis is the representative bivalve with the great ability to enrich for paralytic shellfish toxins (PSTs), however, it has been shown that exposure of Mytilus galloprovincialis to PSTs can induces an inflammatory response in the organism, while its mechanism and effects on toxin metabolism are not clear. In this study, transcriptomics and metabolomics were used to compare the differences in gene expression and metabolite content of purple mussel (M. galloprovincialis) in different periods of Alexandrium catenella bloom, in order to analyze the inflammatory response mechanism of purple mussel under the stress of PSTs. The results show that there are significantly changes in genes and metabolites expressed after exposure to PSTs, including 17 232 differentially expressed genes and 341 differentially expressed metabolites. Based on the association analysis, differentially expressed genes and metabolites are significantly enriched in the arachidonic acid and glutathione metabolic pathways, and genes PLA2G2F, ALOX5, TBXAS1 and metabolites ARA, PGH2, TXA2, LTA4, 5-HETE play important roles in the pro-inflammatory response of the mussel organism; while genes GPX4, CYP2J2 and metabolites 15-HETE and GSH regulate the regression of inflammation in the organism, constituting a complex network of inflammatory regulatory signals. This study reveals that arachidonic acid-related pathways play an important regulatory role in the inflammatory response of the mussel organism, which provides a basis for further insight into the mechanism of the inflammatory network response of the mussel organism under the stress of PSTs.
-
Key words:
- paralytic shellfish toxins /
- Mytilus galloprovincialis /
- metabolism /
- arachidonic acid /
- inflammation
-
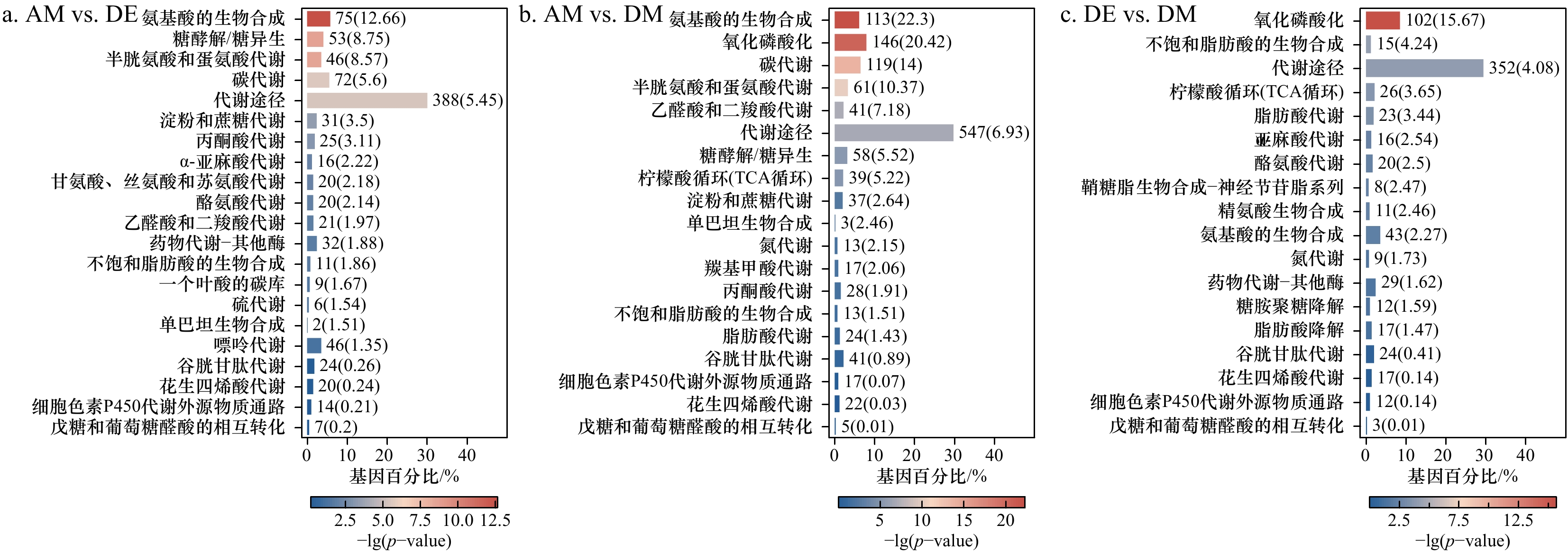
图 2 PSTs暴露后紫贻贝中差异表达基因通路富集条形图
数字代表该通路中具有该作用的基因;括号中的数字表示在通路中具有该作用的基因占总基因的百分比
Fig. 2 Bar graph of enrichment of differentially expressed gene pathways in purple mussels after exposure to PSTs
Numbers represent genes with this function in this pathway; the number in brackets indicates the percentage of genes with this effect in the pathway to the total genes
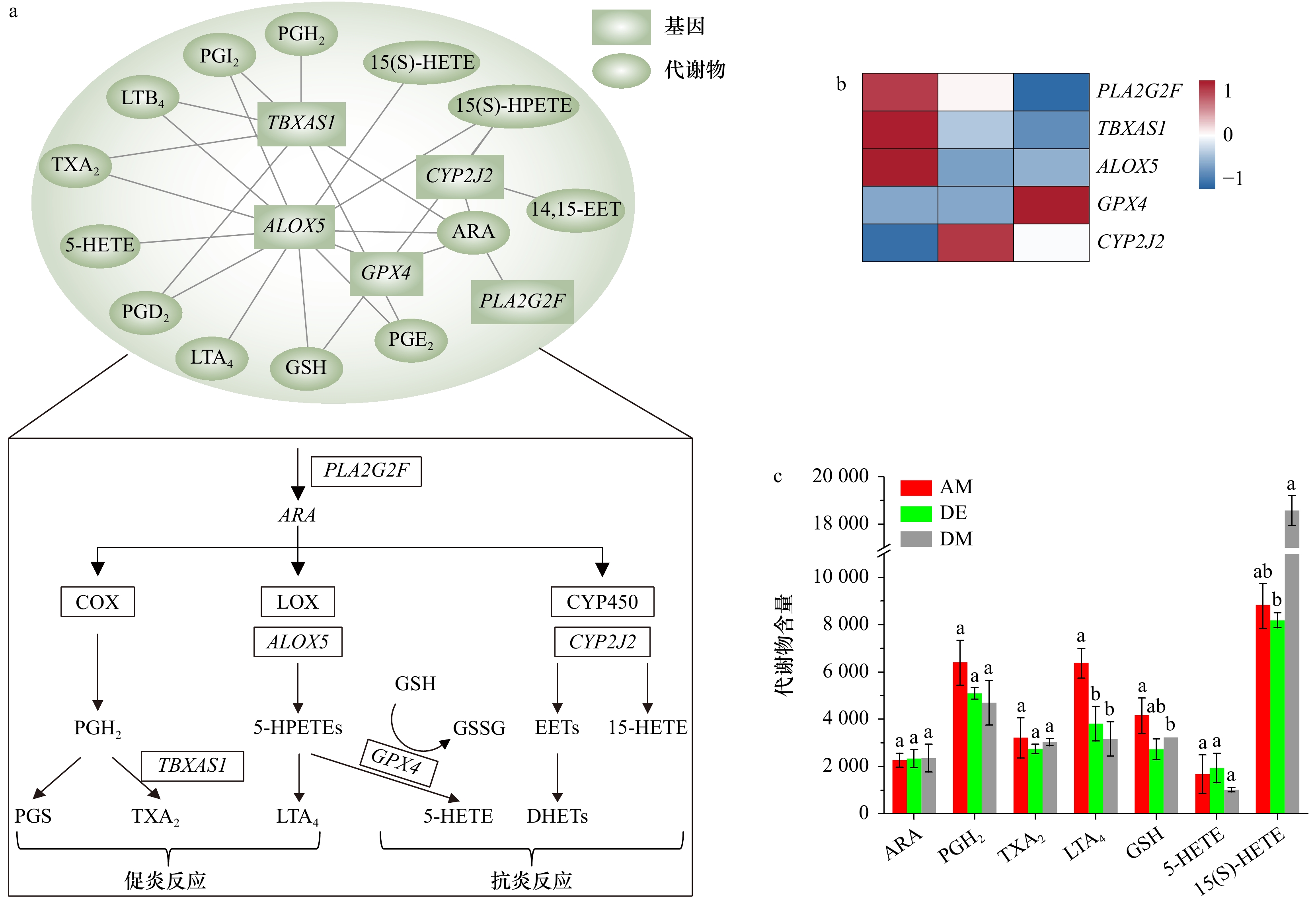
图 5 共富集通路中差异表达基因和差异代谢物相关性网络
a. 差异表达基因和差异代谢物相关性网络图;b. 差异表达基因热图;c. 差异代谢物含量变化,上标字母代表不同代谢物在相同阶段的差异性比较(p < 0.05)
Fig. 5 Correlation network diagram of differentially expressed genes and differential metabolites in co-enrichment pathway
a. Correlation network diagram of differentially expressed genes and differential metabolites; b. heat map of differentially expressed genes; c. group of changes in differential metabolite content, superscript letters represent the differences of different metabolites at the same stage (p < 0.05)
表 1 紫贻贝内脏团转录组测序数据统计
Tab. 1 Statistics of transcriptome sequencing data of purple mussel visceral mass
组别 样品名称 原始数据/bp 高质量序列片段数/bp Q20碱基数量(百分比/%) Q30碱基数量(百分比/%) GC含量(百分比/%) AM AM-1 10 959 472 500 10 911 515 537 10 680 755 661 (97.89) 10 223 214 375 (93.69) 4 828 742 818 (44.26) AM-2 8 125 679 400 8 076 026 204 7 892 394 413 (97.73) 7 542 438 533 (93.39) 3 603 591 162 (44.62) AM-3 8 729 513 400 8 683 772 828 8 497 851 463 (97.86) 8 130 983 080 (93.63) 3 763 289 705 (43.34) DE DE-1 8 073 096 900 8 031 328 826 7 866 098 724 (97.94) 7 535 005 372 (93.82) 3 502 447 726 (43.60) DE-2 8 454 445 800 8 411 111 621 8 253 145 459 (98.12) 7 930 035 993 (94.28) 3 674 190 321 (43.68) DE-3 7 081 375 500 7 048 936 870 6 898 862 938 (97.87) 6 603 158 137 (93.68) 3 180 155 814 (45.12) DM DM-1 8 728 461 300 8 685 090 050 8 516 315 313 (98.06) 8 176 501 926 (94.14) 4 085 887 479 (47.04) DM-2 9 436 620 300 9 395 624 783 9 175 417 519 (97.66) 8 756 082 800 (93.19) 4 250 045 553 (45.23) DM-3 9 723 735 900 9 653 010 060 9 418 303 282 (97.57) 8 980 610 578 (93.03) 4 366 589 867 (45.24) 表 2 显著富集代谢通路代谢物列表
Tab. 2 Significantly enriched metabolic pathway metabolites list
代谢通路 代谢物名称 VIP值 代谢通路 代谢物名称 VIP值 中文名称 简称 中文名称 简称 谷胱甘肽 谷胱甘肽 GSH 2.53 花生四烯酸 8,9-环氧二十碳三烯酸 8,9-EET 1.41 γ-谷氨酰半胱氨酸 γ-GC 1.66 5-羟二十碳四烯酸 5-HETE 1.41 L-谷氨酸 GA 1.07 5,6-环氧-8,11,14-二十碳三烯酸 5,6-E-8,11,14-ET 1.41 半胱氨酸甘氨酸 Cys 1.66 5,6-二羟基二十碳三烯酸 5,6-DHET 1.12 亚精胺 Spe 2.57 20-羟基白三烯B4 20-OH-LTB 4 2.60 花生四烯酸 血栓素A2 TXA2 2.60 19(S)-羟基二十四碳四烯酸 19(S)-HETE 1.41 前列腺素I2 PGI2 2.60 15-羟基-11,12-环氧二十碳三烯酸 15H-11,12-EETA 1.82 前列腺素H2 PGH2 2.60 15(S)-氢过氧二十碳四烯酸 15(S)-HPETE 1.82 前列腺素G2 PGG2 2.52 15(S)-羟基廿碳四烯酸 15(S)-HETE 1.41 前列腺素E2 PGE2 2.60 12(S)-羟过氧化二十四碳四烯酸 12(S)-HPETE 1.82 前列腺素D2 PGD2 2.60 11-羟基-14,15-环氧二十碳三烯酸 11H-14,15-EETA 1.82 亚精胺 Spe 2.57 11,14,15-三羟基二十三碳烯酸 11,14,15-THETA 1.05 白三烯B4 LTB4 1.82 11,12-环氧二十碳三烯酸 11,12-EET 1.41 白三烯A4 LTA4 1.24 11,12-二羟基乙酸三油酸 11,12-DiHETrE 1.12 花生四烯酸 ARA 1.59 11,12,15-三羟基二十碳三烯酸 11,12,15-THETA 1.05 -
[1] Xu Houguo, Meng Xiaoxue, Wei Yuliang, et al. Arachidonic acid matters[J]. Reviews in Aquaculture, 2022, 14(4): 1912−1944. doi: 10.1111/raq.12679 [2] 冀倩倩. 花生四烯酸ARA对仿刺参生长及免疫相关功能的影响[D]. 大连: 大连工业大学, 2021.Ji Qianqian. Effects of arachidonic acid on the growth and immune function of Apostichopus japonicus[D]. Dalian: Dalian Polytechnic University, 2021. [3] 王成强, 王际英, 李宝山, 等. 饲料中花生四烯酸含量对刺参生长性能、抗氧化能力及脂肪酸代谢的影响[J]. 中国水产科学, 2018, 25(3): 555−566. doi: 10.3724/SP.J.1118.2018.17344Wang Chengqiang, Wang Jiying, Li Baoshan, et al. Effects of dietary arachidonic acid on the growth performance, antioxidant capacity, and fatty acid metabolism of sea cucumber ( Apostichopus japonicus)[J]. Journal of Fishery Sciences of China, 2018, 25(3): 555−566. doi: 10.3724/SP.J.1118.2018.17344 [4] Tian Jingjing, Lei Caixia, Ji Hong, et al. Dietary arachidonic acid decreases the expression of transcripts related to adipocyte development and chronic inflammation in the adipose tissue of juvenile grass carp, Ctenopharyngodon idella[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2019, 30: 122−132. doi: 10.1016/j.cbd.2019.02.006 [5] Furne M, Holen E, Araujo P, et al. Cytokine gene expression and prostaglandin production in head kidney leukocytes isolated from Atlantic cod ( Gadus morhua) added different levels of arachidonic acid and eicosapentaenoic acid[J]. Fish & Shellfish Immunology, 2013, 34(3): 770−777. [6] Cho S Y, Kwon Y K, Nam M, et al. Integrated profiling of global metabolomic and transcriptomic responses to viral hemorrhagic septicemia virus infection in olive flounder[J]. Fish & Shellfish Immunology, 2017, 71: 220−229. [7] Abshirini M, Coad J, Wolber F M, et al. Green-lipped (greenshell™) mussel ( Perna canaliculus) extract supplementation in treatment of osteoarthritis: a systematic review[J]. Inflammopharmacology, 2021, 29(4): 925−938. doi: 10.1007/s10787-021-00801-2 [8] 赵利斌, 王鑫磊, 黄旭雄, 等. 饲料中花生四烯酸水平对凡纳滨对虾免疫相关基因表达及抗菌能力的影响[J]. 水产学报, 2016, 40(5): 763−775.Zhao Libin, Wang Xinlei, Huang Xuxiong, et al. Effects of dietary arachidonic acid on the immune-related gene expressions and Vibrio-resistant ability in Litopenaeus vannamei[J]. Journal of fisheries of China, 2016, 40(5): 763−775. [9] 王成强, 王际英, 黄炳山, 等. 饲料花生四烯酸水平对珍珠龙胆石斑鱼幼鱼生长性能、抗氧化能力、血清生化指标以及肝脏和肌肉脂肪酸组成的影响[J]. 动物营养学报, 2018, 30(9): 3567−3580. doi: 10.3969/j.issn.1006-267x.2018.09.027Wang Chengqiang, Wang Jiying, Huang Bingshan, et al. Effects of dietary arachidonic acid level on growth performance, antioxidant ability, serum biochemical parameters and fatty acid composition in liver and muscle of juvenile hybrid grouper ( Epinephelus fuscoguttatus♀× Epinephelus lanceolatus♂)[J]. Chinese Journal of Animal Nutrition, 2018, 30(9): 3567−3580. doi: 10.3969/j.issn.1006-267x.2018.09.027 [10] 张圆琴, 徐后国, 曹林, 等. 饲料中花生四烯酸对发育前期大菱鲆亲鱼性类固醇激素合成的影响[J]. 水产学报, 2017, 41(4): 588−601.Zhang Yuanqin, Xu Houguo, Cao Lin, et al. Effects of dietary arachidonic acid on the sex steroid hormone synthesis in turbot broodstock before maturation[J]. Journal of Fisheries of China, 2017, 41(4): 588−601. [11] 于仁成, 罗璇. 我国近海有毒藻和藻毒素的研究现状与展望[J]. 海洋科学集刊, 2016(1): 155−166.Yu Rencheng, Luo Xuan. Status and research perspectives on toxic algae and Phycotoxins in the coastal waters of China[J]. Studia Marina Sinica, 2016(1): 155−166. [12] Nakatani T, Masayama A, Kiyota K, et al. Comparison between mouse bioassay and HILIC-MS/MS for quantification of paralytic shellfish toxin in Japanese basket clams and mussels caught off coastal Osaka Bay in Japan[J]. Food Additives & Contaminants: Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 2021, 38(11): 1969−1983. [13] Faggio C, Tsarpali V, Dailianis S. Mussel digestive gland as a model tissue for assessing xenobiotics: an overview[J]. Science of the Total Environment, 2018, 636: 220−229. doi: 10.1016/j.scitotenv.2018.04.264 [14] Lassudrie M, Hégaret H, Wikfors G H, et al. Effects of marine harmful algal blooms on bivalve cellular immunity and infectious diseases: a review[J]. Developmental & Comparative Immunology, 2020, 108: 103660. [15] Braga A C, Pereira V, Marçal R, et al. DNA damage and oxidative stress responses of mussels Mytilus galloprovincialis to paralytic shellfish toxins under warming and acidification conditions—Elucidation on the organ-specificity[J]. Aquatic Toxicology, 2020, 228: 105619. doi: 10.1016/j.aquatox.2020.105619 [16] Lushchak V I. Environmentally induced oxidative stress in aquatic animals[J]. Aquatic Toxicology, 2011, 101(1): 13−30. doi: 10.1016/j.aquatox.2010.10.006 [17] Even Y, Pousse E, Chapperon C, et al. Physiological and comparative proteomic analyzes reveal immune defense response of the king scallop Pecten maximus in presence of paralytic shellfish toxin (PST) from Alexandrium minutum[J]. Harmful Algae, 2022, 115: 102231. doi: 10.1016/j.hal.2022.102231 [18] Freitas R, Marques F, De Marchi L, et al. Biochemical performance of mussels, cockles and razor shells contaminated by paralytic shellfish toxins[J]. Environmental Research, 2020, 188: 109846. doi: 10.1016/j.envres.2020.109846 [19] Geret F, Serafim A, Barreira L, et al. Effect of cadmium on antioxidant enzyme activities and lipid peroxidation in the gills of the clam Ruditapes decussatus[J]. Biomarkers, 2002, 7(3): 242−256. doi: 10.1080/13547500210125040 [20] García-Lagunas N, De Jesus Romero-Geraldo R, Hernández-Saavedra N Y. Changes in gene expression and histological injuries as a result of exposure of Crassostrea gigas to the toxic dinoflagellate Gymnodinium catenatum[J]. Journal of Molluscan Studies, 2016, 82(1): 193−200. [21] Balbi T, Auguste M, Ciacci C, et al. Immunological responses of marine bivalves to contaminant exposure: contribution of the-omics approach[J]. Frontiers in Immunology, 2021, 12: 618726. doi: 10.3389/fimmu.2021.618726 [22] Dong Zhicheng, Chen Yan. Transcriptomics: advances and approaches[J]. Science China Life Sciences, 2013, 56(10): 960−967. doi: 10.1007/s11427-013-4557-2 [23] Dong Chenfan, Wu Haiyan, Zheng Guanchao, et al. Transcriptome analysis reveals MAPK/AMPK as a key regulator of the inflammatory response in PST detoxification in Mytilus galloprovincialis and Argopecten irradians[J]. Toxins, 2022, 14(8): 516. doi: 10.3390/toxins14080516 [24] Hu Boyang, Li Moli, Yu Xiaohan, et al. Diverse expression regulation of Hsp70 genes in scallops after exposure to toxic Alexandrium dinoflagellates[J]. Chemosphere, 2019, 234: 62−69. doi: 10.1016/j.chemosphere.2019.06.034 [25] García-Lagunas N, Romero-Geraldo R, Hernández-Saavedra N Y. Genomics study of the exposure effect of Gymnodinium catenatum, a paralyzing toxin producer, on Crassostrea gigas’ defense system and detoxification genes[J]. PLoS One, 2013, 8(9): e72323. doi: 10.1371/journal.pone.0072323 [26] Cappello T, Giannetto A, Parrino V, et al. Baseline levels of metabolites in different tissues of mussel Mytilus galloprovincialis (Bivalvia: Mytilidae)[J]. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 2018, 26: 32−39. doi: 10.1016/j.cbd.2018.03.005 [27] Blanco J, Mariño C, Martín H, et al. Anatomical distribution of diarrhetic shellfish poisoning (DSP) toxins in the mussel Mytilus galloprovincialis[J]. Toxicon, 2007, 50(8): 1011−1018. doi: 10.1016/j.toxicon.2007.09.002 [28] Qi Hongqing, Liu Ying, Jian Fanjie, et al. Effects of dietary arachidonic acid (ARA) on immunity, growth and fatty acids of Apostichopus japonicus[J]. Fish & Shellfish Immunology, 2022, 127: 901−909. [29] Carrier III J K, Watanabe W O, Harel M, et al. Effects of dietary arachidonic acid on larval performance, fatty acid profiles, stress resistance, and expression of Na+/K+ ATPase mRNA in black sea bass Centropristis striata[J]. Aquaculture, 2011, 319(1/2): 111−121. [30] Gerdol M, Moreira R, Cruz F, et al. Massive gene presence-absence variation shapes an open pan-genome in the Mediterranean mussel[J]. Genome Biology, 2020, 21(1): 275. doi: 10.1186/s13059-020-02180-3 [31] Murgarella M, Puiu D, Novoa B, et al. A first insight into the genome of the filter-feeder mussel Mytilus galloprovincialis[J]. PLoS One, 2016, 11(3): e151561. [32] Yu Rencheng, Zhang Qingchun, Liu Yang, et al. The dinoflagellate Alexandrium catenella producing only carbamate toxins may account for the seafood poisonings in Qinhuangdao, China[J]. Harmful Algae, 2021, 103: 101980. doi: 10.1016/j.hal.2021.101980 [33] Zhou Zhiwei, Luo Mingdu, Chen Xi, et al. Ion mobility collision cross-section atlas for known and unknown metabolite annotation in untargeted metabolomics[J]. Nature Communications, 2020, 11(1): 4334. doi: 10.1038/s41467-020-18171-8 [34] Bouallegui Y. Immunity in mussels: an overview of molecular components and mechanisms with a focus on the functional defenses[J]. Fish & Shellfish Immunology, 2019, 89: 158−169. [35] Ciacci C, Betti M, Canonico B, et al. Specificity of anti- Vibrio immune response through p38 MAPK and PKC activation in the hemocytes of the mussel Mytilus galloprovincialis[J]. Journal of Invertebrate Pathology, 2010, 105(1): 49−55. doi: 10.1016/j.jip.2010.05.010 [36] Wu Haiyan, Dong Chenfan, Zheng Guanchao, et al. Formation mechanism and environmental drivers of Alexandrium catenella bloom events in the coastal waters of Qinhuangdao, China[J]. Environmental Pollution, 2022, 313: 120241. doi: 10.1016/j.envpol.2022.120241 [37] Castrec J, Soudant P, Payton L, et al. Bioactive extracellular compounds produced by the dinoflagellate Alexandrium minutum are highly detrimental for oysters[J]. Aquatic Toxicology, 2018, 199: 188−198. doi: 10.1016/j.aquatox.2018.03.034 [38] Tan K, Sun Yizhou, Zhang Hongkuan, et al. Effects of harmful algal blooms on the physiological, immunity and resistance to environmental stress of bivalves: special focus on paralytic shellfish poisoning and diarrhetic shellfish poisoning[J]. Aquaculture, 2023, 563: 739000. doi: 10.1016/j.aquaculture.2022.739000 [39] Hanna V S, Hafez E A A. Synopsis of arachidonic acid metabolism: a review[J]. Journal of Advanced Research, 2018, 11: 23−32. doi: 10.1016/j.jare.2018.03.005 [40] El Kertaoui N, Lund I, Betancor M B, et al. Dietary DHA and ARA level and ratio affect the occurrence of skeletal anomalies in pikeperch larvae ( Sander lucioperca) through a regulation of immunity and stress related gene expression[J]. Aquaculture, 2021, 544: 737060. doi: 10.1016/j.aquaculture.2021.737060 [41] Gagné F, Auclair J, Peyrot C, et al. The influence of zinc chloride and zinc oxide nanoparticles on air-time survival in freshwater mussels[J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2015, 172−173: 36−44. [42] Powell W S, Rokach J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid[J]. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 2015, 1851(4): 340−355. [43] Lin Yan, Lu Xinchen, Qiu Xinghua, et al. Arachidonic acid metabolism and inflammatory biomarkers associated with exposure to polycyclic aromatic hydrocarbons[J]. Environmental Research, 2022, 212: 113498. doi: 10.1016/j.envres.2022.113498 [44] Calder P C. Eicosanoids[J]. Essays in Biochemistry, 2020, 64(3): 423−441. doi: 10.1042/EBC20190083 [45] Liu Yingjie, Chen Zhongxiang, Li Shanwei, et al. Multi-omics profiling and biochemical assays reveal the acute toxicity of environmental related concentrations of Di-(2-ethylhexyl) phthalate (DEHP) on the gill of crucian carp ( Carassius auratus)[J]. Chemosphere, 2022, 307: 135814. doi: 10.1016/j.chemosphere.2022.135814 [46] Škugor S, Škugor A, Todorčević M, et al. Exposure to lipopolysaccharide induces immune genes in cultured preadipocytes of Atlantic salmon[J]. Fish & Shellfish Immunology, 2010, 29(5): 817−824. [47] Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation[J]. Science, 1983, 220(4597): 568−575. doi: 10.1126/science.6301011 [48] Boltaña S, Tridico R, Teles M, et al. Lipopolysaccharides isolated from Aeromonas salmonicida and Vibrio anguillarum show quantitative but not qualitative differences in inflammatory outcome in Sparus aurata (Gilthead seabream)[J]. Fish & Shellfish Immunology, 2014, 39(2): 475−482. [49] Tallima H. Clarification of arachidonic acid metabolic pathway intricacies[J]. ACS Omega, 2021, 6(24): 15559−15563. doi: 10.1021/acsomega.1c01952 [50] Guengerich F P. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity[J]. Chemical Research in Toxicology, 2001, 14(6): 611−650. doi: 10.1021/tx0002583 [51] Han J, Park J S, Park Y, et al. Effects of paralytic shellfish poisoning toxin-producing dinoflagellate Gymnodinium catenatum on the marine copepod Tigriopus japonicus[J]. Marine Pollution Bulletin, 2021, 163: 111937. doi: 10.1016/j.marpolbul.2020.111937 [52] Wei Xiaomeng, Lu Miyu, Duan Guofang, et al. Responses of CYP450 in the mussel Perna viridis after short-term exposure to the DSP toxins-producing dinoflagellate Prorocentrum lima[J]. Ecotoxicology and Environmental Safety, 2019, 176: 178−185. doi: 10.1016/j.ecoenv.2019.03.073 [53] Maha I F, Xie Xiao, Zhou Suming, et al. Skin metabolome reveals immune responses in yellow drum Nibea albiflora to Cryptocaryon irritans infection[J]. Fish Shellfish Immunology, 2019, 94: 661−674. doi: 10.1016/j.bcp.2018.10.007 [54] Painefilú J C, Bianchi V A, Krock B, et al. Effects of paralytic shellfish toxins on the middle intestine of Oncorhynchus mykiss: glutathione metabolism, oxidative status, lysosomal function and ATP-binding cassette class C (ABCC) proteins activity[J]. Ecotoxicology and Environmental Safety, 2020, 204: 111069. doi: 10.1016/j.ecoenv.2020.111069 [55] Li Cong, Deng Xiaobing, Xie Xiaowen, et al. Activation of glutathione peroxidase 4 as a novel anti-inflammatory strategy[J]. Frontiers in Pharmacology, 2018, 9: 1120. doi: 10.3389/fphar.2018.01120 -



 下载:
下载: